Boston Scientific accounts are for healthcare professionals only.

SEISMIQ™ Intravascular Lithotripsy System
Reimbursement
Configure or select a product to continue to order
- Overview
- Technical specifications
- Ordering information
- Clinical data
Precision at Full Force
The next-generation IVL system designed to put you in control of therapy delivery.
Level up with targeted IVL therapy.

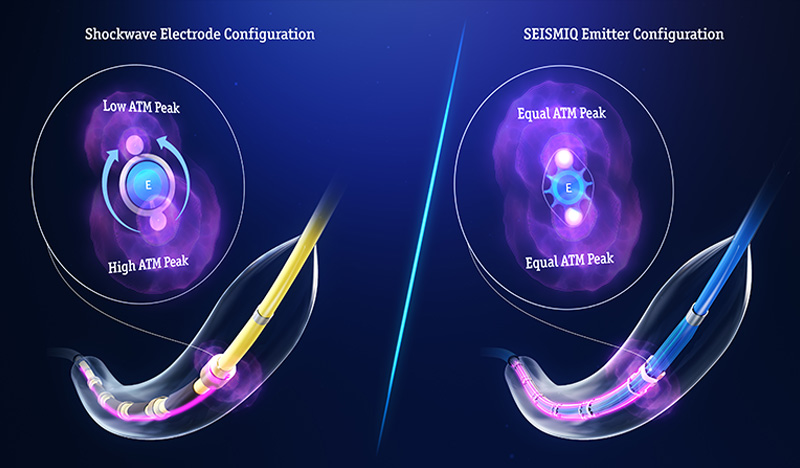
How SEISMIQ works
SEISMIQ delivers targeted acoustic pressure to modify calcified arterial lesions.
SEISMIQ’s advanced laser energy source is distributed through optical fibers, then converted to acoustic pressure waves by generating a plasma event when the laser energy hits each emitter's metallic backstop within the balloon. The initial plasma plume fractures the calcium, while a secondary cavitation bubble further expands the fractures.
Why choose SEISMIQ
Get the most from every pulse
Technical information
SEISMIQ console specifications
| Feature | |
|---|---|
| Built-in-test | Built-in-tests and monitors are designed to detect designated malfuntions of subsystems within the console. The console is designed to interrupt therapy delivery in the event a malfunction is detected. |
| Classification product | Class I Safety Medical Equipment per IEC 60601-1 |
| Classification applied parts | Type CF Defribrillation Proof |
| Data log | The console logs important software and hardware events for troubleshooting. No patient information is managed or recorded by the SEISMIQ IVL console. |
| Dimensions | Height: 1.5 m Width: 0.8 m Depth: 1.1 m |
| Environment | Operating Temperature: 15°C to 35°C Operating Relative Humidity: 15% to 70% non-condensing Storage Temperature: -30°C to 60°C Storage Relative Humidity: 15% to 90% Atmospheric Pressure: 794 mbar to 1013 mbar |
| Mobility | Product is designed to be movable |
| Power | 100–240 VAC 50/60 Hz |
| Relative pressure measurement | Measurement range: 0–30 ATM Measurement accuracy: ± 0.2 ATM |
| Splash resistance | Protected from condensation |
| Weight | 190 lbs or 85 kg |
| Fuses | 2 X T 10AH 250V |
SEISMIQ catheter specifications
| Feature | |
|---|---|
| Catheter working length | 145cm |
| Balloon length | 60mm |
| Balloon giameters | 3.5–8.0mm |
| Coated length | Distal 15cm |
| Crossing profile | .064in |
| Emitter stations | 5 pairs |
| GW compatibility | .014in |
| Sheath compatibility (largest size) | 6F |
| Speed | 3.33 Hz |
| Total number of cycles | 12 |
Ordering information
Review the ordering detail information below. Contact a rep to order
| Description | Working Length | Guidewire (in) | UPN | GTIN |
|---|---|---|---|---|
| 3.5 mm x 60 mm | 145 cm | 0.014 | FG-006912 | 00850056723008 |
| 4.0 mm x 60 mm | 145 cm | 0.014 | FG-006913 | 00850056723015 |
| 5.0 mm x 60 mm | 145 cm | 0.014 | FG-006915 | 00850056723039 |
| 6.0 mm x 60 mm | 145 cm | 0.014 | FG-006917 | 00850056723053 |
| 7.0 mm x 60 mm | 145 cm | 0.014 | FG-006919 | 00850056723077 |
| 8.0 mm x 60 mm | 145 cm | 0.014 | FG-006921 | 00850056723091 |
Clinical data
RESTORE clinical trials
Feature | ATK Intent-to-Treat (ITT) = 95 As Treated (AT) = 95 | BTK Intent-to-Treat (ITT) = 20As Treated (AT) = 18 |
Design | ------------------- Prospective, non-randomized, multicenter study ------------------- | |
Sites | 10 sites | 3 sites |
IVL Use | 95/95 (100%) | 18/18 (100%)* |
| Lesion Characteristics* | ||
Calcification Severity (PARC) | 91.6% | 83.3% |
Avg. Lesion Length (mm) | 96.0 ± 37.5 | 68.6 ± 39.6 |
Initial Diameter Stenosis (%) | 93.7 ± 7.2 | 91.4 ± 8.8 |
CTO‡ | 32 (33.7%) | 6 (30.0%)1 |
Final Diameter Stenosis (%) | 21.2 ± 8.4 | 22.8 ± 11.5 |
Acute Gain (mm) | 3.2 ± 0.9 | 1.3 ± 0.6 |
Procedural Details |
|
|
Pre-Dilatation | 25 (26.3%) | 4 (22.2%) |
Post-Dilatation | 17 (17.9%) | 3 (16.7%) |
Stent | 3 (3.2%) | 0 (0%) |
Primary Safety Endpoint | 100% freedom from MAE at 30 days | 100% freedom from MAE at 30 days |
Primary Efficacy Endpoint | 100% with residual diameter stenosis | 100% with acute reduction in percent diameter stenosis of target lesion |
Conclusion | Met all primary endpoints; | Demonstrated safety and effectiveness |
* As treated group
‡ Calculated by Angiographic Core Lab
1. Preliminary data presented at VEITH 2024 & VIVA 2024: (1) Brodmann, M. (2024). A New More Complex Device for Treating Calcified Arterial Lesions with Lithotripsy (From Bolt Medical: How Does It Work and Advantages, VEITH 2024. (2) Zeller, T (2024) Laser and Optics-Based Peripheral Intravascular Lithotripsy System for the Treatment of Above-the-Knee and Below-the-Knee Calcified Lesions. Results of the RESTORE ATK and RESTORE BTK trials. VIVA 2024.