Boston Scientific accounts are for healthcare professionals only.

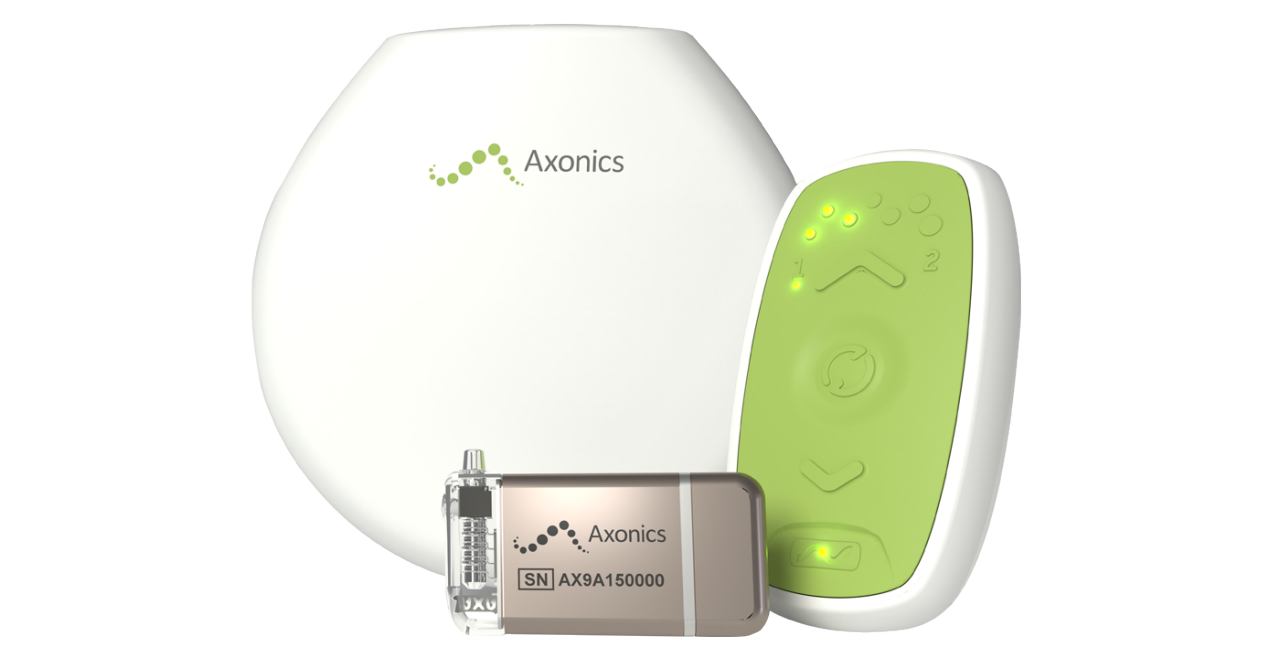
Axonics Sacral Neuromodulation System™
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical data
- Technical specifications
- Ordering information
- Training
- Resources
No compromises, just long-lasting therapy
With Axonics Sacral Neuromodulation, the choice between a rechargeable and recharge-free neurostimulator comes without compromising therapy longevity.
Why choose Axonics SNM?
Axonics has improved the patient and provider experience with sacral neuromodulation therapy, allowing patients to receive long-lasting relief.
Axonics F15™ SNM System: Longest recharge-free battery life in SNM
The Axonics F15 System has redefined the recharge-free experience by delivering longer battery life and a truly recharge-free system.
About the system
- Battery life of 17.6 years at 1 mA* and over 20 years at lower energy settings
- Patients do not recharge any system components
- Thin design — only 10 cc in size
- Delivers constant current stimulation to reduce therapy amplitude adjustments
*Stimulation settings at frequency 14 Hz, pulse width 210 μs, impedance 1200 ohms

Axonics R20™ SNM System: Revolutionizing the neurostimulator recharge experience
The Axonics R20 System is designed to last 20+ years and offers the longest recharge interval available in SNM, providing patients the freedom to live more and recharge less.
About the system
- Proprietary titanium-ceramic design, allowing for safe and efficient charging
- Typical recharging interval every 6 to 10 months
- Charges wirelessly through the skin
- Designed to last 20+ years
- Miniaturized device, only 5 cc in volume
- Delivers constant current stimulation to reduce therapy amplitude adjustments
Ease of charging
In a clinical study of patients who completed a 2-year follow-up:
- 91% rated the recharging experience as "easy".
- 94% found charging frequency and duration to be "ACCEPTABLE."1
Intuitive remote control
For both the F15 recharge-free and R20 rechargeable SNM systems:
- Designed to be pocket-sized and easy to use
- One-handed operation to connect to implantable neurostimulator (INS)
- Allows increase or decrease of stimulation
Patented tined lead design
- Patented helical-tined design to mitigate lead migration
- Visible and radiopaque markers for lead implantation
- Compatible with both F15 recharge-free and R20 rechargeable systems
MRI conditionally safe
Broad MRI access:
- 1.5 T and 3 T Full-Body Coil
- 1.5 T and 3 T Head Coil
- 1.5 T and 3 T Upper and Lower Extremity
- 1.5 T Full-Body Coil with Open Impedances
- 3 T Full-Body Coil with Lead Fragments
Under certain conditions. For more information visit, www.axonics.com/mri.
SmartMRI™ technology:
- Designed to simplify the process for your patients to get an MRI
- Using the Remote Control, the MR technician can easily confirm if a patient's Axonics System is ready to undergo a full-body MRI
- No additional office visit needed prior to MRI
Smart programming
A smart clinician programmer with a proprietary algorithm designed to simplify the programming process
- Proprietary algorithm that generates programming recommendations based on intra-operative responses and lead placement
- Designed to reduce the need to adjust therapy
Compatible system components to streamline the therapy experience
- Clinician programmer to test stimulation during lead placement and program therapy settings
- External trial system offers basic and advanced trial options that are lightweight and discreet
- One tined lead compatible with both F15 and R20 systems, further improving the therapy experience and offering flexibility in clinical decision making
The Axonics SNM Trial System
Axonics SNM offers the ability to trial the therapy and assess symptom improvement prior to having your patient undergo the full implant procedure. The trial is not specific to a system and utilizes an external trial simulator (ETS).

Two types of trials
Basic Trial
A Basic SNM Trial, or peripheral nerve evaluation (PNE), utilizes a temporary lead and ETS.
The temporary lead is placed via a minimally invasive outpatient procedure.
Advanced Trial
An Advanced Trial utilizes a tined lead that is connected to the ETS via a percutaneous extension.
This option offers a longer trial period to test the efficacy of the therapy with the permanent tined lead.
Clinical data
2021, Neurourology Urodynamics, Pezzella et al.
Two‐year outcomes of the ARTISAN‐SNM study for the treatment of urinary urgency incontinence using the Axonics rechargeable sacral neuromodulation system
The ARTISAN-SNM study is a 129-patient, single-arm, prospective, multicenter, pivotal clinical study approved under an FDA Investigational Device Exemption (IDE) to evaluate the safety and efficacy of Axonics Therapy for urinary dysfunction. The study was conducted in a total of 19 centers, 14 in the United States, and 5 in Western Europe.
2020, Neurourology Urodynamics, Blok et al.
Two‐year safety and efficacy outcomes for the treatment of overactive bladder using a long‐lived rechargeable sacral neuromodulation system
The RELAX-OAB study was a 51-patient, post-market clinical follow-up study in Europe designed to test the safety and efficacy of Axonics Therapy for the treatment of overactive bladder. The study was conducted across 7 European centers. Patients in the RELAX-OAB study have completed long-term follow-up out to 2 years, showing sustained efficacy and safety of Axonics Sacral Neuromodulation Therapy.
Publication list
2020, Journal of Urology, McCrery et al.
2018, Neurourology Urodynamics, Blok et al.
2018, Neurourology Urodynamics, Blok et al.
2018, Neurourology Urodynamics, Elterman
2018, Neurourology Urodynamics, Blok et al.
The system features of the Axonics F15 and the Axonics R20
Axonics F15
| Type |
|---|
| Recharge-free |
| Recharging interval |
|---|
| N/A |
| Battery life |
|---|
| 10 to 20 years of battery life |
| Neurostimulator case |
|---|
| 10 cc Titanium |
| MRI labeling |
|---|
| 1.5 T and 3 T Full-Body Coil MRI |
| Stimulation type |
|---|
| Constant current |
Axonics R20
| Type |
|---|
| Rechargeable |
| Recharging interval |
|---|
| Every 6 to 10 months |
| Battery life |
|---|
| 20+ years |
| Neurostimulator case |
|---|
| 5 cc Titanium-ceramic |
| MRI labeling |
|---|
| 1.5 T and 3 T Full-Body Coil MRI |
| Stimulation type |
|---|
| Constant current |
Ordering information
| Product Description | UPN |
|---|---|
| Clinician Programmer | 2501 |
| Non-Rechargeable F15 Neurostimulator | 4101 |
| Rechargeable R20 Neurostimulator | 5101 |
| Tined Lead | 1201 |
| Tined Lead Implant Kit | 1801 |
| Remote Control | 2301 |
| Charging System | 1401 |
| External Trial Stimulator | 1601 |
| PNE Lead Implant Kit | 1701 |
| PNE Lead | 1901 |
| Percutaneous Extension | 9009 |
| Stimulation Ground Cable 6PK | 9003 |
| Torque Wrench 10PK | 9004 |
| Needle Test Stimulation Cable - box of 6 | 9006 |
| Tined Lead Stimulation Cable - box of 6 | 9007 |
| Dilator and Sheath - box of 6 | 9011 |
| Power Supply | 9015 |
| Foramen Needle, Long - box of 6 | 9001-L |
| Foramen Needle, Short - box of 6 | 9001-S |
| Curved Belt Extended | 9108-E |
| Curved Belt Regular | 9108-R |
Online medical training and education courses
The EDUCARE online platform makes medical education and training relevant, comprehensive, personal, and accessible. Register to access procedural videos, case studies, and training resources.
Assessing your patients for sacral neuromodulation
Urinary or bowel dysfunctions can be a difficult topic for patients to discuss. Establishing an easy way to assess whether your patients are suffering from these conditions can help identify their needs. Incorporate a bladder and bowel health assessment into your regular exams and help your patients discuss their symptoms with you.
Guiding your patients through the care pathway
First- and second-line treatments may not always provide the symptom relief your patients are seeking to improve their quality of life. Patients may attempt conservative treatment options, but if symptom improvement is not adequate, they may become discouraged and not seek further treatment, especially if they are not aware that there are other advanced treatment options available.
Educating your patients about the care pathway may help motivate them to stay engaged in their treatment and to follow up if symptoms are not improved.
Questions?
We are here to help answer any questions you may have about Axonics SNM.
Reference:
- Pezzella A, McCrery R, Lane F, et al. Two-year outcomes of the ARTISAN-SNM study for the treatment of urinary urgency incontinence using the Axonics rechargeable sacral neuromodulation system. Neurourol Urodyn. 2021;40:714–721.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician trained in use of surgical mesh for repair of stress urinary incontinence.
All trademarks are the property of their respective owners.
All images are the property of Boston Scientific.