Boston Scientific accounts are for healthcare professionals only.
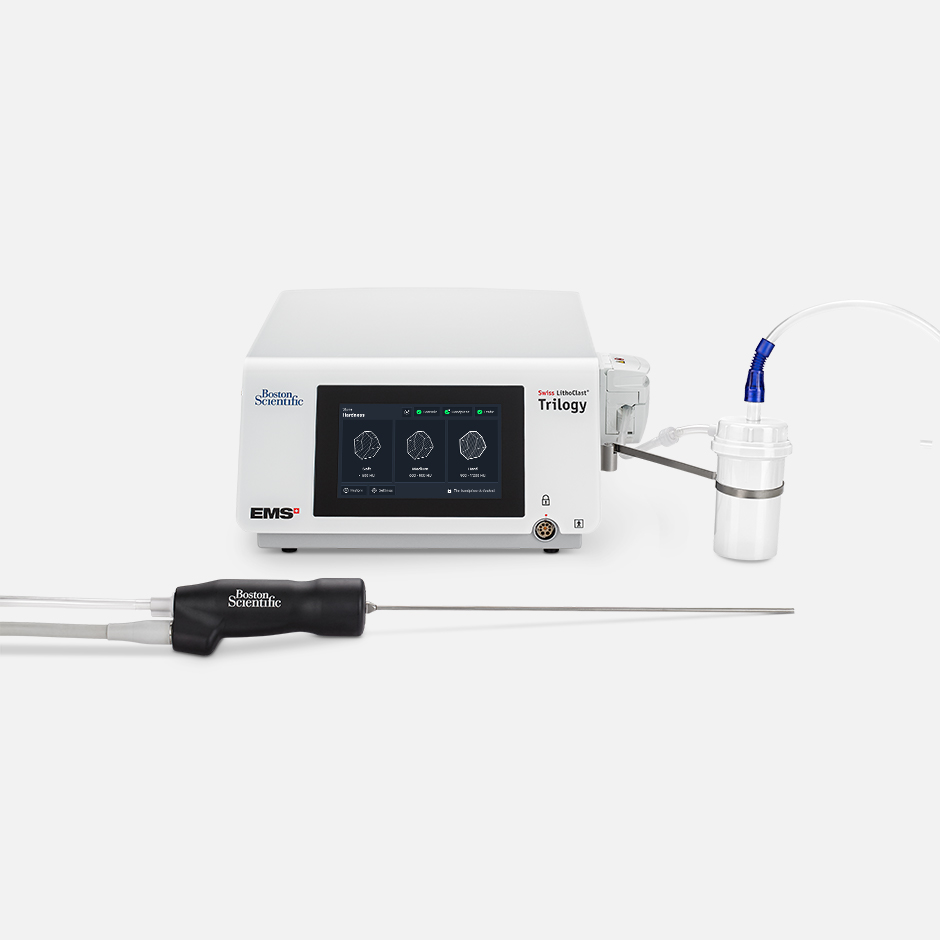
Swiss LithoClast® Trilogy Max Lithotripter
Reimbursement
Configure or select a product to continue to order
- Overview
- Clinical Data
- Ordering Information
- Training
- Resources
Enhanced ergonomics. Proven legacy of performance.*
The Trilogy Max Lithotripter features the enhancements physicians requested, while providing the same efficient performance as the first-generation Trilogy.
The dual-energy Trilogy Lithotripter is designed to deliver controlled ultrasonic and ballistic energy concurrently or independently with simultaneous suction through a single probe for removal of kidney stones, ureter stones, and bladder stones.
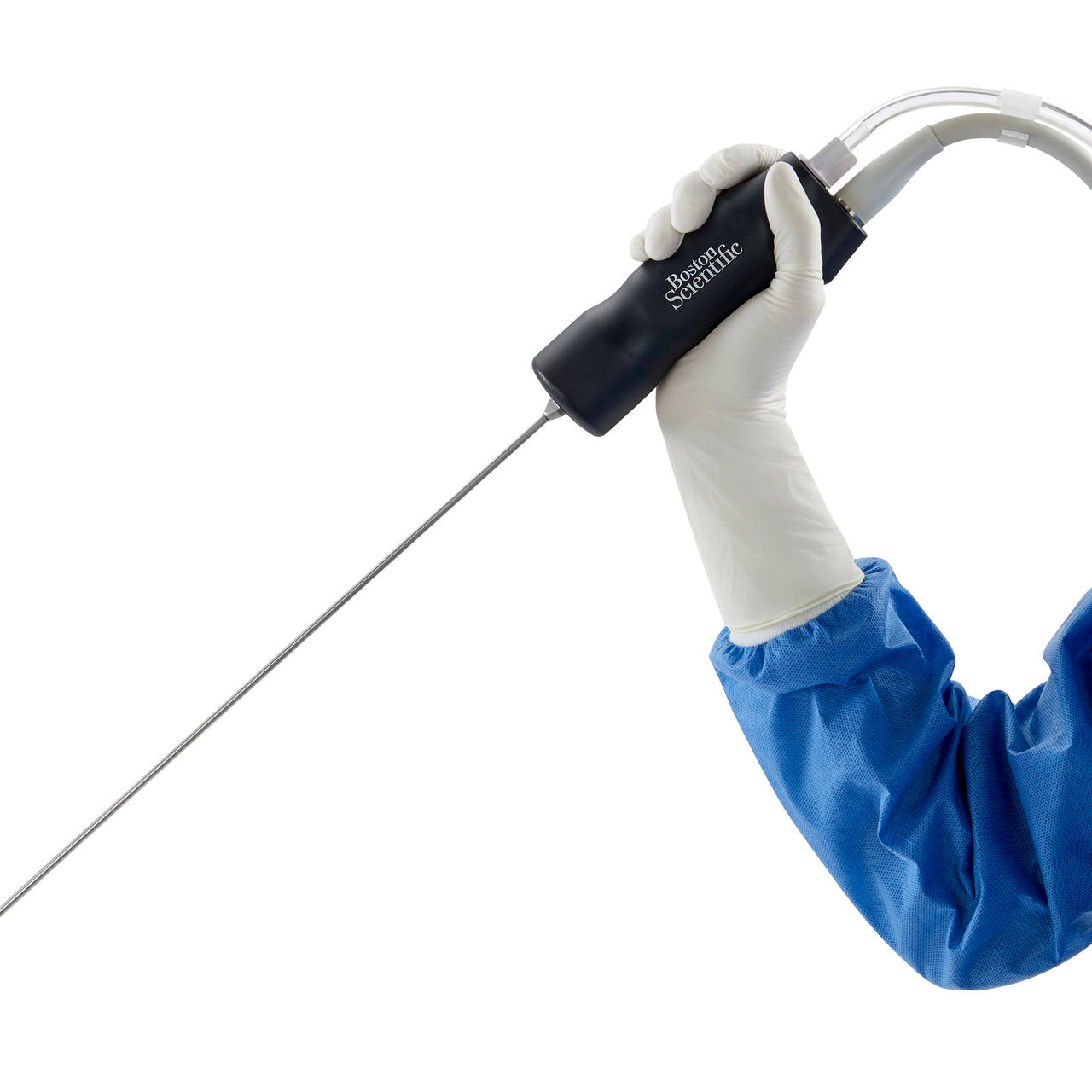
Enhanced ergonomic handpiece
Feel more comfortable throughout stone removal procedures with an ergonomic handpiece that’s:
- Designed to be lightweight and comfortable to use in prone and supine patient positioning4
- 25% lighter, with a smaller profile4
- Fitted with a new cord that reduces the torque effect on the handle by 30%4
- Designed to reduce user strain and fatigue due to less torque and weight4

Improved software and interface
Utilize upgraded software and interface enhancements including:
- Three presets as well as the ability to customize settings for each case
- An optimized cooling system designed to reduce device downtime due to handpiece overheating during a procedure, potentially improving efficiency
Easier setup for picture-in-picture so the physician only has to look at one screen

Wider, wireless foot pedal
Experience more stability with a wider foot pedal that’s designed to:
- Reduce unintentional pedal movement
- Ease foot placement and activation
- Reduce cord-related hazards and clutter
Additional wireless features include:
- Automatic pairing
Powered by a standard AA battery with a low battery indicator to minimize case disruption
See how it works
September 2021, Mary Ann Liebert Inc., Tim Large et al.
Prospective, randomized controlled trial showed significantly fewer perioperative malfunctions, less suction occlusion, and 49%*** fewer secondary procedures due to residual stone fragments with the first-generation Trilogy compared to the Olympus ShockPulse-SE Lithotripter.
April 2020, World Journal of Urology, Bader, M.J., Eisel, M., Strittmatter, F. et al.
Bench study demonstrated that the first-generation Trilogy removed hard stones up to 48%** faster, on average, compared to the Olympus ShockPulse-SE Lithotripter.
May 2018, Journal of Endourology, Evan C Carlos et al.
Bench study demonstrates that the first-generation Trilogy mean clearance time was nearly 2 times faster than that of Olympus ShockPulse-SE and 4 times faster than that of LithoClast Select (p<0.01).1
Swiss LithoClast Trilogy Max System Kit and Accessories
| UPN | GTIN | Included in Trilogy Max System Kit | Description |
|---|---|---|---|
| M006840202K0 | 07613353311582 | Yes | Swiss LithoClast Trilogy Max System Kit |
| M0068402010 | 07613353125707 | Yes | Swiss LithoClast Trilogy Console |
| M006840213 | 07613353222413 | Yes | Swiss LithoClast Trilogy Max Handpiece Kit (Reusable Torque Wrench included in Handpiece Kit) |
| M0068402050 | 07613353125806 | Yes | Swiss LithoClast Trilogy Foot Switch |
| M0068403590 | 07613353133351 | Yes | Swiss LithoClast Trilogy Reusable Torque Wrench |
| M0068401810 | 07613353125820 | Yes | Stone Catcher Holder |
| M0068401800 | 07613353125769 | Yes | HDMI/DVI cable (10m) |
| M0068401820 | 07613353125837 | Yes | Filling Bottle |
| M0068402830 | 07613353125844 | Yes | Bottle of Demineralized Water (1 L) |
| M0068401850 | 07613353125721 | Yes | Power Cord (US - 6m) |
| M0068401860 | 07613353165246 | Yes | Swiss LithoClast Trilogy System IFU (English) |
| M0068401890 | 07613353163983 | Yes | Quick Guide |
| M0068401890 | 07613353125790 | Yes | Cleaning Rod for Trilogy/Trilogy Max Handpiece |
| M0068401790 | 07613353125899 | No | Trilogy/Trilogy Max Handpiece Suction Connector |
| M0068401640 | 07613353164720 | No | Steri Holder Trilogy |
| M0068401760 | 07613353125912 | No | Swiss Lithoclast Trilogy Cart |
| M0068402150 | 0761335321415 | No | Swiss LithoClast Trilogy Wireless Foot Switch Kit |
| Single-Use Accessories (Optional) | |||
| M0068402981 | 07613353126384 | No | Stone Catcher for Trilogy (Bx5) |
| M0068402061 | 07613353132118 | No | Double Suction Bags Set (Bx5) |
| M0068402071 | 07613353133931 | No | Suction Bag 5l (Bx10) |
Swiss LithoClast Trilogy/Trilogy Max Single-Use Probe Kits
Single-Use Probe Kits include a Probe and Stone Catcher
| UPN | GTIN | Description | Color Code |
|---|---|---|---|
| M0068403550 | 07613353123703 | 3.9mm x 440mm Trilogy Probe Kit | Blue |
| M0068403540 | 07613353123697 | 3.9mm x 350mm Trilogy Probe Kit | Blue |
| M0068403530 | 07613353123727 | 3.4mm x 445mm Trilogy Probe Kit | Green |
| M0068403520 | 07613353123710 | 3.4mm x 340mm Trilogy Probe Kit | Green |
| M0068403510 | 07613353123734 | 1.9mm x 341mm Trilogy Probe Kit | Yellow |
| M0068403500 | 07613353162702 | 1.5mm x 440mm Trilogy Probe Kit | Orange |
| M0068403490 | 07613353123772 | 1.1mm x 625mm Trilogy Probe Kit | Red |
| M0068403480 | 07613353123765 | 1.1mm x 520mm Trilogy Probe Kit | Red |
| M0068403470 | 07613353123758 | 1.1mm x 425mm Trilogy Probe Kit | Red |
Mini PCNL with Trilogy
William Atallah, MD, and Mantu Gupta, MD, discuss the case of a 56-year-old female with kidney stones treated with mini PCNL using first-generation Trilogy.

Advances in Third Generation Dual-Energy Lithotripsy
Drs. Preminger, Miller, Sur, and Mr. Wiseman discuss the third generation dual-energy lithotripter Trilogy including basic science, the European and US experiences, and Mini PCNL with Trilogy probes.
Online medical training and education courses
The EDUCARE online platform makes healthcare education and training more relevant, more comprehensive, more personal, and more accessible. Register to access a library of procedural videos, case studies, training resources, and events.
These healthcare professional resources are provided as an educational service for use by practicing physicians and allied healthcare professionals. This download center is not intended for direct use by patients or consumers.
Resource downloads:
Product Photography:
Stay up to date
Sign up to receive the latest news and updates about Boston Scientific stone solutions.
Footnotes:
*First-generation Trilogy
** Boston Scientific calculation: (23.79–46.04)/46.04, 23.79 seconds (Trilogy) vs. 46.04 seconds (ShockPulse-SE), p<0.01
*** Boston Scientific calculation: (17.7–34.7)/34.7, 17.7% (Trilogy) vs. 34.7% (ShockPulse-SE), p=0.005
References:
- Carlos EC, Wollin DA, Winship BB, et al. In vitro comparison of a novel single probe dual-energy lithotripter to current devices. J Endourol. 2018;32:534–540.
- Bader MJ, Eisel M, Strittmatter F, et al. Comparison of stone elimination capacity and drilling speed of endoscopic clearance lithotripsy devices. World J Urol. 2021;39:563–569.
- Large T, Nottingham CU, Brinkman E, et al. Multi-institutional prospective randomized control trial of novel intracorporeal lithotripters: ShockPulse-SE vs. Trilogy Trial. J Endourol. 2021;35:1326–1332.
- Data on file.
Bench test results may not necessarily be indicative of clinical performance.
Physicians pictured in social media posts are or may be paid consultants for Boston Scientific.
Swiss LithoClast® Trilogy Max Lithotripter is manufactured by E.M.S. Electro Medical Systems S.A. and distributed by Boston Scientific Corporation.
Caution: U.S. Federal law restricts this device to sale by or on the order of a physician.
All images are property of Boston Scientific. All trademarks are the property of their respective owners.