Implant Technique
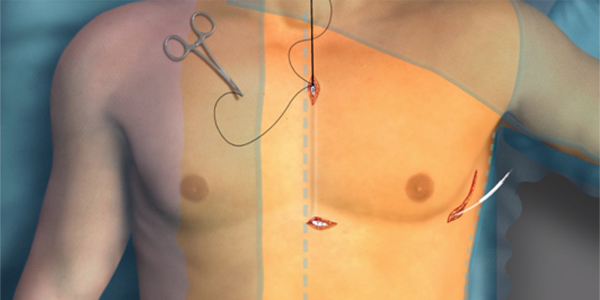
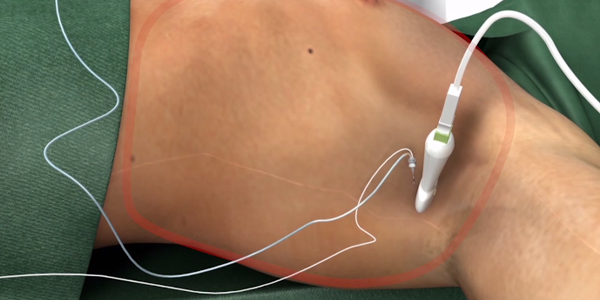
Anaesthesia
As the implant technique and clinical data for S-ICD continues to advance, it is important to understand the evolving anaesthesia options for the management of patients undergoing S-ICD placement. There are multiple proven periprocedural sedation and anaesthesia options for S-ICD implants, including general anaesthesia, monitored anesthesia care, regional anaesthesia (Truncal Blocks), and non-anaesthesia administered sedation and analgesia.1
On-demand interactive webinar
Cadaver demonstration of surgical anatomy and loco-regional blocks for S-ICD implant
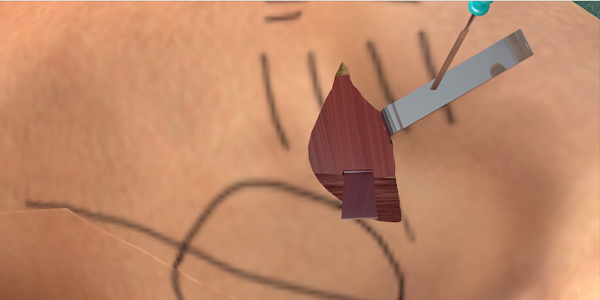
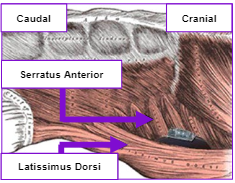
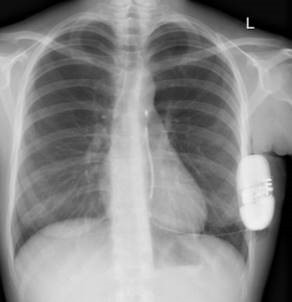
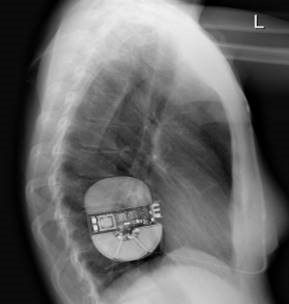
Intermuscular Technique
The intermuscular technique positions the pulse generator postero-laterally between the anterior surface of the serratus anterior and the posterior surface of the latissimus dorsi and helps drive a predictable and consistent implant experience.
The intermuscular technique has many benefits.2-7
- Optimal position for DFT and impedance measurements
- Reduced risk of pocket complications (erosion and infection)
- Reduced device migration
- Consistency in implant technique
- Enhanced patient comfort as the device is protected by the muscle layer
- Excellent cosmetic outcomes
- Intermuscular placement can be particularly beneficial in low and high BMI patients
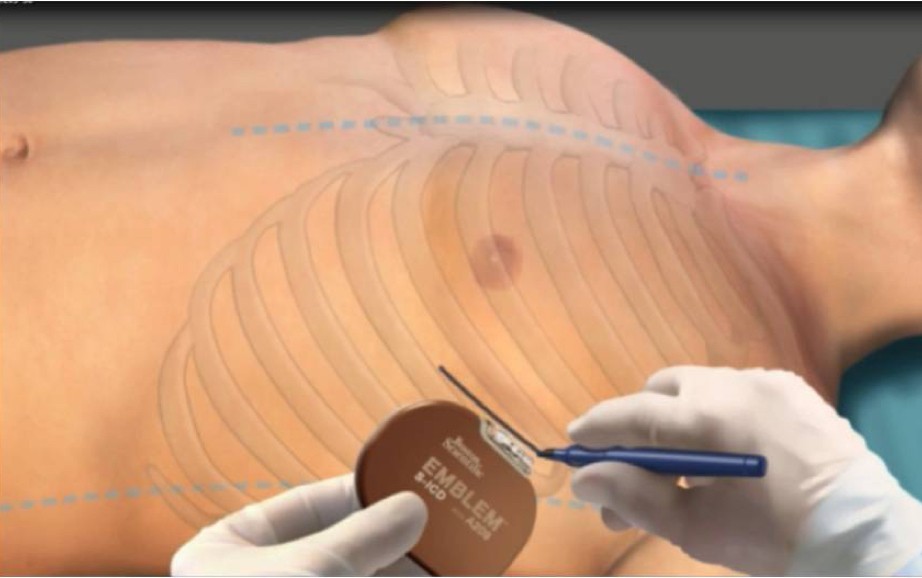
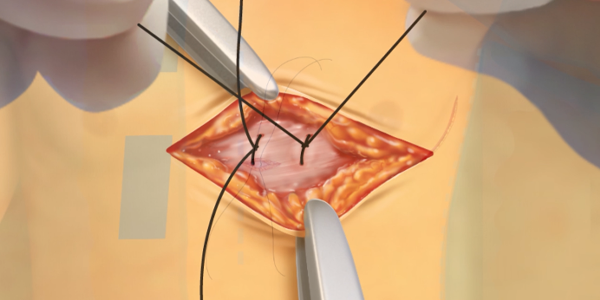
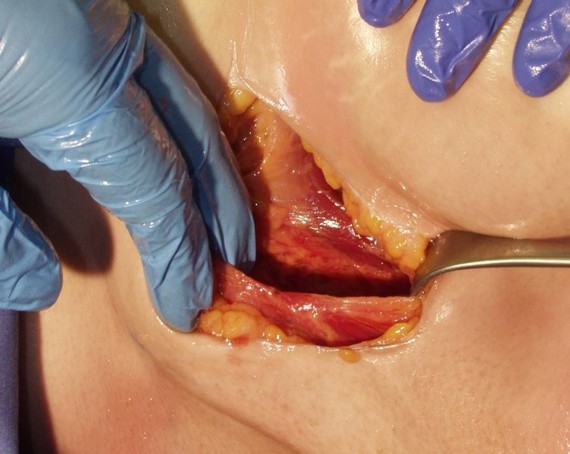
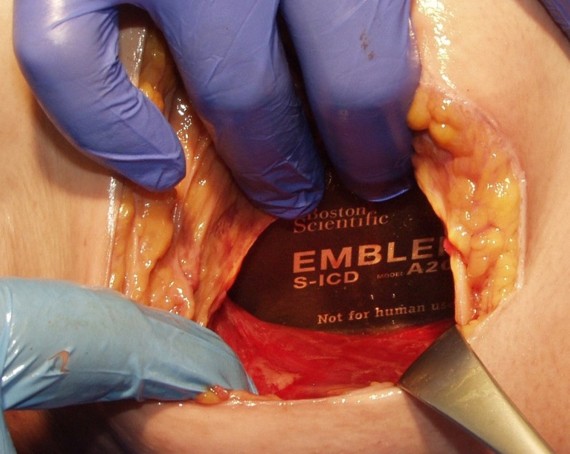
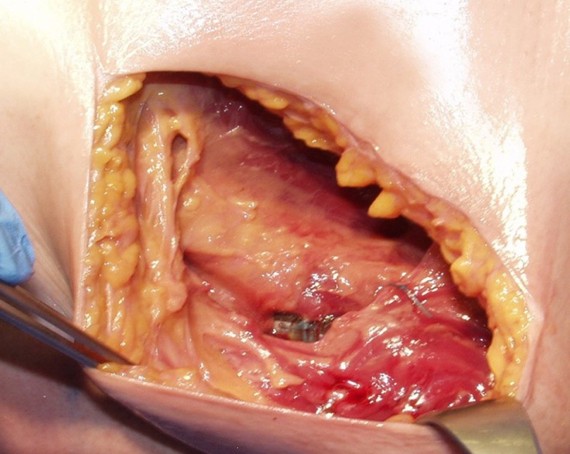
Formation of the Intermuscular Pocket
From the incision, the subcutaneous tissue is dissected directly down to the fascia, the contour of the chest wall is followed to create the pocket. The pulse generator is secured to the fascia of the serratus anterior.2-7
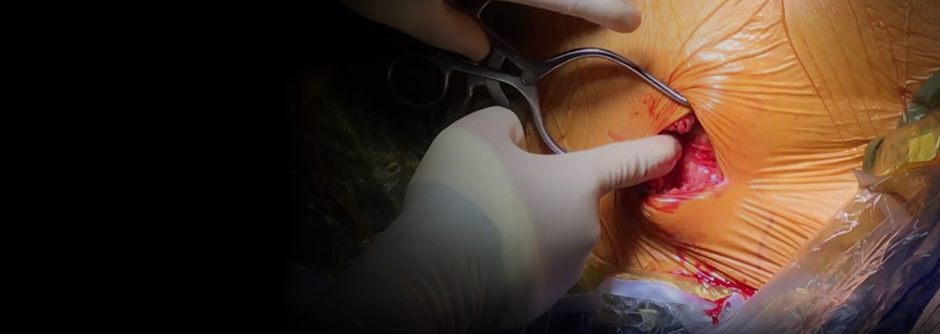
Intermuscular Pocket
Formation Video
Excellent Cosmetic Outcomes and Optimal S-ICD Placement
Intermuscular Implant Technique for S-ICD
S-ICD Replacements
When replacing an S-ICD device, it is critical to assess the current position of the S-ICD system and determine corrective actions. Watch the video below to better understand how to approach an S-ICD replacement while using best practices.

Best Practices
on S-ICD Replacements

Education & Training
Why S-ICD?
See how S-ICD helps protect patients at risk for sudden cardiac death while also eliminating the risk of TV-ICD lead complications.
















.png)
