VANTAGE Clinical Trial 1,2
Key Resources
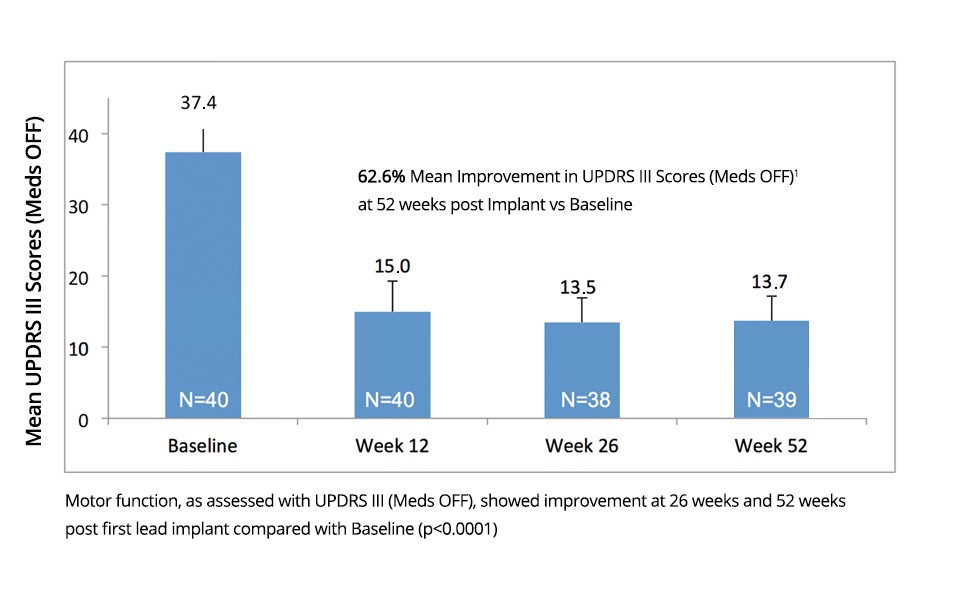
Results Demonstrate:
 | Vercise DBS system showed significant improved motor function (p<0.0001), as assessed by a 62.6% (±19.8) mean reduction in UPDRS III scores at Week 26, which was sustained up to Week 52 post-lead placement (p < 0.0001). |
 | All secondary endpoints including quality of life, medication usage and global impression of change (subject/clinician) were statistically significant. |
 | The Vercise rechargeable battery and charging system was well tolerated by subjects. |
 | At 26 and 52 weeks post lead implant, approximately 70% of programs had current fractionalized over 2 or more contacts. |
Baseline (n=40) | 12-Week Mean Change from Baseline [95% CI](n=40) | 26-Week Mean Change from Baseline [95% CI](n=38) | 52-Week Mean Change from Baseline [95% CI] (n=39) | p-value(F) |
|---|---|---|---|---|
| 37.4 ± 8.9 | -22.3 ± 8.1 [-24.9, -19.7] | -23.8 ± 10.6 [-27.3, -20.3] | -23.7 ± 8.8 [-26.6, -20.9] | <0.0001 |
Methods
Forty (40) subjects with Parkinson’s disease were implanted bilaterally with Boston Scientific’s multiple source current-controlled DBS system (Vercise) in the STN at 6 European Centers. Subjects’ devices were activated 2 – 18 days after implant and followed up at Weeks 12, 21, 26 and Year 1 post first lead placement. All implanting surgeons were carefully trained on using the system.
The primary endpoint of the study was the change in UPDRS III scores stim ON/meds OFF at 6 mos. compared with pre-operative scores (Baseline meds OFF). All raters were systematically trained in UPDRS assessments. Other assessments to quantify motor improvements such as CAPSIT motor tests, Tremor Rating Scale, and Dyskinesia Rating Scale were also administered. Data collected also included assessments to evaluate changes in quality of life, such as PDQ-39, Short Form Health Survey (SF-36) and modified Schwab and England Scale. Subject motor diaries were collected over 3 consecutive days. Adverse events were recorded.
Key Inclusion Criteria
 | Diagnosis of bilateral idiopathic PD ≥ 5 years |
 | Modified Hoehn and Yahr in the OFF state ≥ 2 |
 | UPDRS III ≥ 30 off meds that improved by ≥ 33% with medications |
 | Appropriate surgical candidate for DBS |
The Vantage Study and the Vercise DBS System
- Timmermann, Lars et al. Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson's disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. The Lancet Neurology, Volume 14, Issue 7, 693 - 701.
- Timmerman L., Alesch, A., et.al. VANTAGE trial: A prospective, multi-center trial evaluating Deep Brain Stimulation with a new multiple-source, constant-current rechargeable system (VerciseTM) in Parkinson’s disease. Movement Disorder Society, Poster June 2013.
















