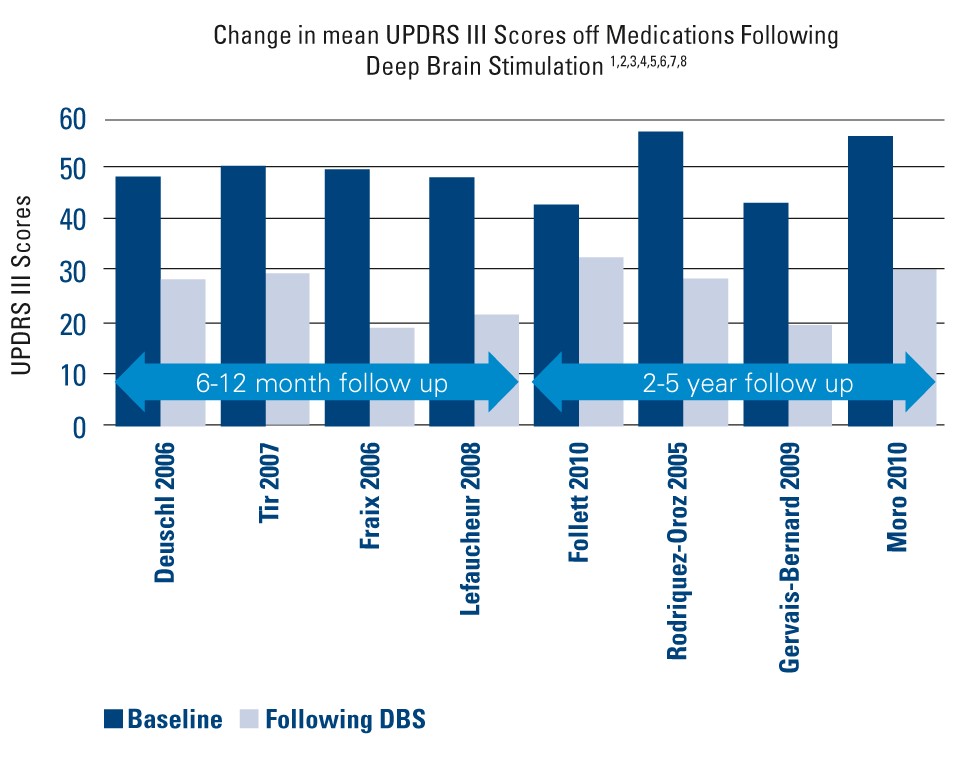
Clinical Effectiveness of DBS for Parkinson's Disease
Primary | Study Design | Sample Size | Follow Up | Change in Mean UPDRS III Scores | % Improvement in UPDRS III & PDQ-39 Scores |
|---|---|---|---|---|---|
| Deuschl 2006¹ | Randomized pairs trial | 78 | 6 mos. | • Baseline: 48 ± 12.3 • Post DBS: 28.3 ± 14 | • 41% improvement in UPDRS III • 25% improvement in PDQ-39 |
| Tir 2007² | Prospective, single-center study | 103 | 12 mos. | • Baseline: 50 ± 16 • Post DBS: 29 ± 11.5 | • 42% improvement in UPDRS III |
| Fraix 2006³ | Prospective, multi-center | 95 | 12 mos. | Baseline: 49.2 ± 16.4 • Post DBS: 19.4 ± 11.5 | • 57% improvement in UPDRS III |
Lefaucheur 2008⁴ | Single-center study | 54 | 12 mos. | Baseline: 48.2 ± 16.1 • Post DBS: 21.4 ± 8.2 | • 56% improvement in UPDRS III |
| Follett 2010⁵ | Multi-center, randomized, blinded | 299 | 24 mos. | STN • Baseline: 43 ± 15 • Post DBS: 32.1 ± 15.6 GPi • Baseline: 41.8 ± 13.1 • Post DBS: 30 ± 14.2 | • 25.3% improvement in UPDRS III • Improvement in 6 of 8 subscales |
Rodriquez- Oroz 2005⁶ | Multi-center study | 69 | 3-4 yrs. | STN • Baseline: 56.7 ± 15.7 • Post DBS: 28.6 ± 15.7 GPi • Baseline: 51.7 ± 13.6 • Post DBS: 31.7 ± 12.8 | • 50% improvement in UPDRS III with STN and 39% improvement with GPi at 3-4 yrs. |
Gervais- Bernard 2009⁷ | Prospective, single-center | 23 | 5 yrs. | Baseline: 43.11 ± 14.04 • Post DBS: 19.52 ± 7.17 | • 55% improvement in UPDRS III |
| Moro 2010⁸ | Nonrandomized, prospect, blinded, multicenter study | 51 | 5-6 yrs. | STN • Baseline: 56 ± 2.7 • Post DBS: 30.1 ± 2.5 GPi • Baseline: 52.2 ± 3.5 • Post DBS: 32.6 ± 4.6 | • 45.4 (STN) to 20% (GPi) improvement in UPDRS III |
- Deuschl G , Schade-Brittinger C et al. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. N Engl J Med 2006;355:896-908.
- Tir M, Exhaustive, one-year follow-up of subthalamic nucleus deep brain stimulation in a large, single-center cohort of Parkinson’s patients, Neurosurgery 61:297–305, 2007.
- Fraix V, Houeto JL, Lagrange C et al. Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2006;77:443–449.
- Lafaucheur JP, Gurruchaga JM, Pollin B et al. Outcome of Bilateral Subthalamic Nucleus Stimulation in the Treatment of Parkinson’s Disease: Correlation with Intra-Operative Multi-Unit Recordings but Not with the Type of Anaesthesia. Eur Neurol 2008;60:186–199.
- Follett KA, Weaver FM, Stern M et al. Pallidal versus Subthalamic Deep-Brain Stimulation for Parkinson’s Disease. N Engl J Med 2010;362:2077-91.
- Rodriguez-Oroz MC, Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up, Brain (2005), 128, 2240–2249.
- Gervais-Bernard H, Xie-Brustolin J, Mertens P et al, Bilateral subthalamic nucleus stimulation in advanced Parkinson’s disease: Five year follow-up. J Neurol (2009) 256:225–233.
- Moro E, Lozano A, Pollak P et al. Long-Term Results of a Multicenter Study on Subthalamic and Pallidal Stimulation in Parkinson’s Disease. Movement Disorders Vol. 25, No. 5, 2010, pp. 578–586 1.