AGENT™
Drug-Coated Balloon
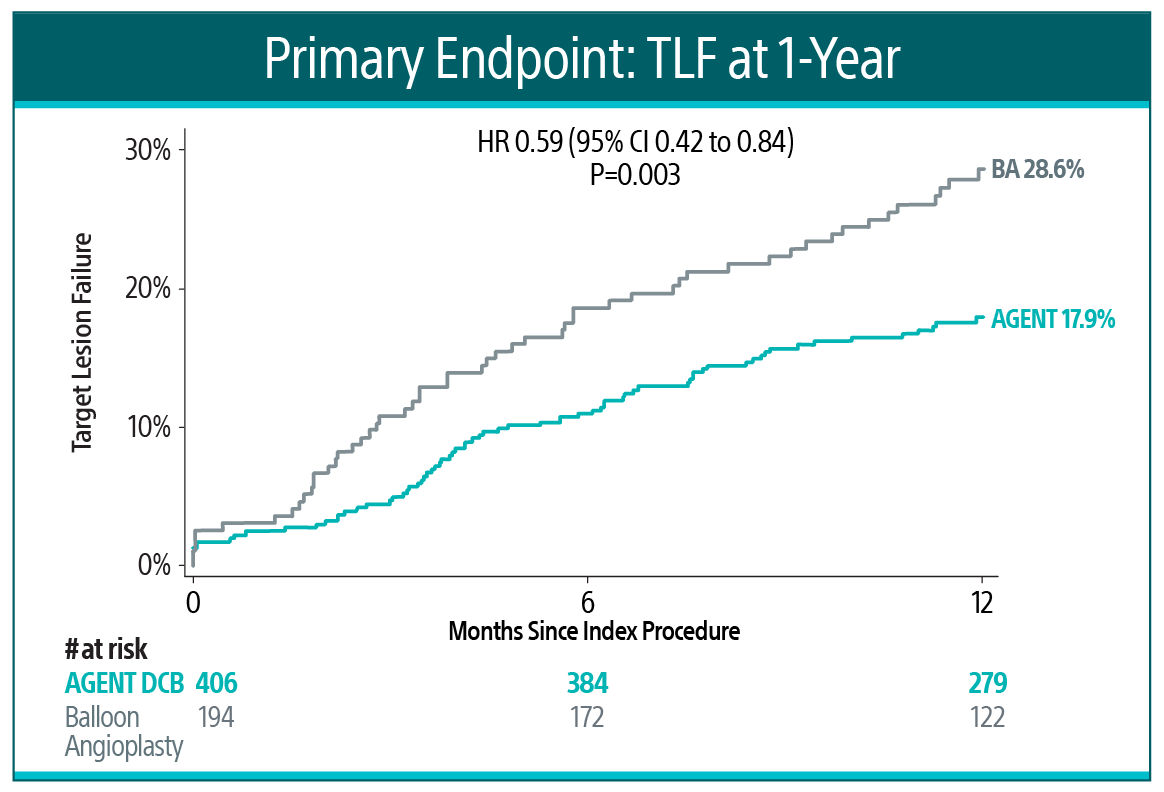
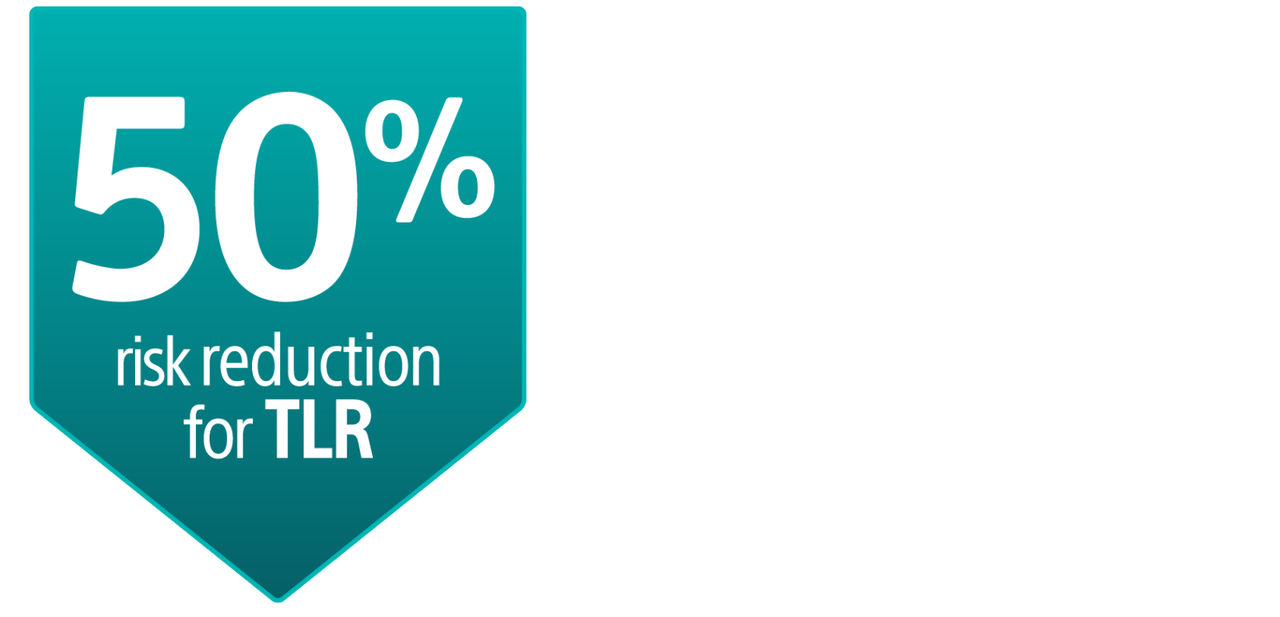
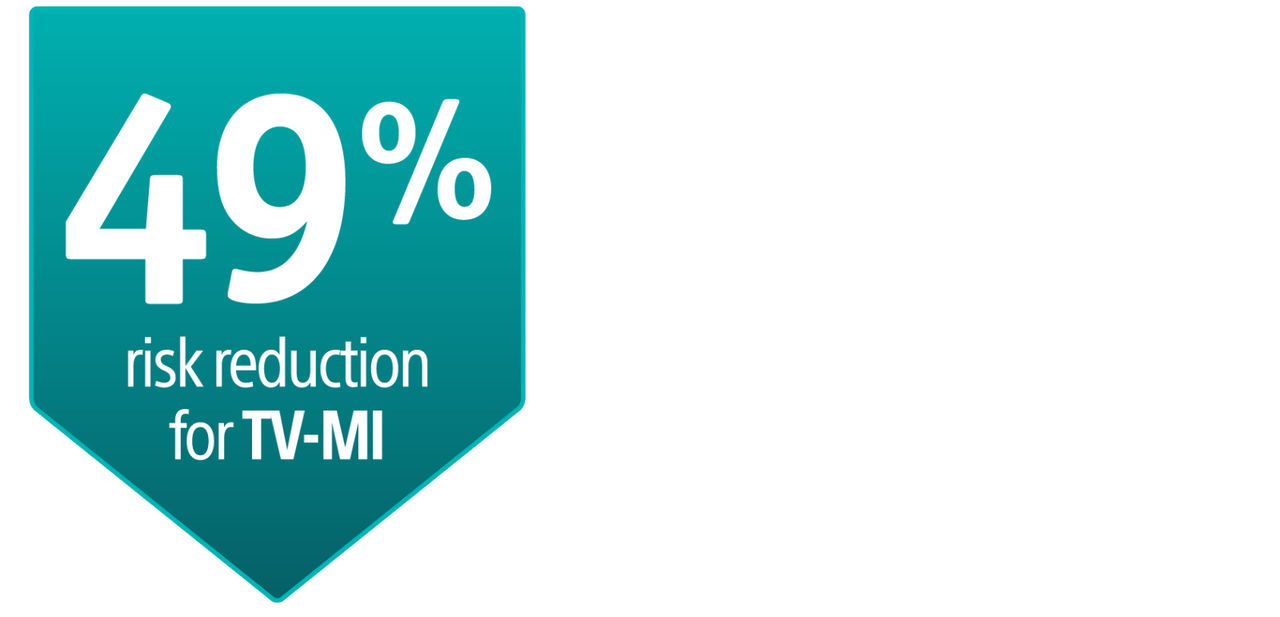
The AGENT IDE Clinical Trial showed that AGENT DCB is superior to conventional balloon angioplasty in reducing target lesion failure for the treatment of coronary in-stent restenosis (ISR).1
Expanding Clinical Evidence Beyond ISR
100,000+ patients have been treated with AGENT DCB globally, with over 4,400 patients evaluated or currently undergoing evaluation with AGENT DCB.3 Patients are at the center of everything we do at Boston Scientific as we continue to research and advance science for life. That’s why we sponsored the AGENT DCB STANCE Trial.
This trial is designed to assess the safety and effectiveness of the AGENT DCB compared to the standard of care – percutaneous coronary intervention (PCI) treatment with drug-eluting stents (DES) and/or balloon angioplasty – in patients with de novo coronary lesions.
Patients enrolling in the trial must have a de novo target lesion located in a native coronary artery. The study is examining de novo lesions in small vessels, bifurcations, and long lesions. The primary endpoint is Target Lesion Failure (TLF) at 12 months.
Visit clinicaltrials.gov for more information on trial sites and expected completion.
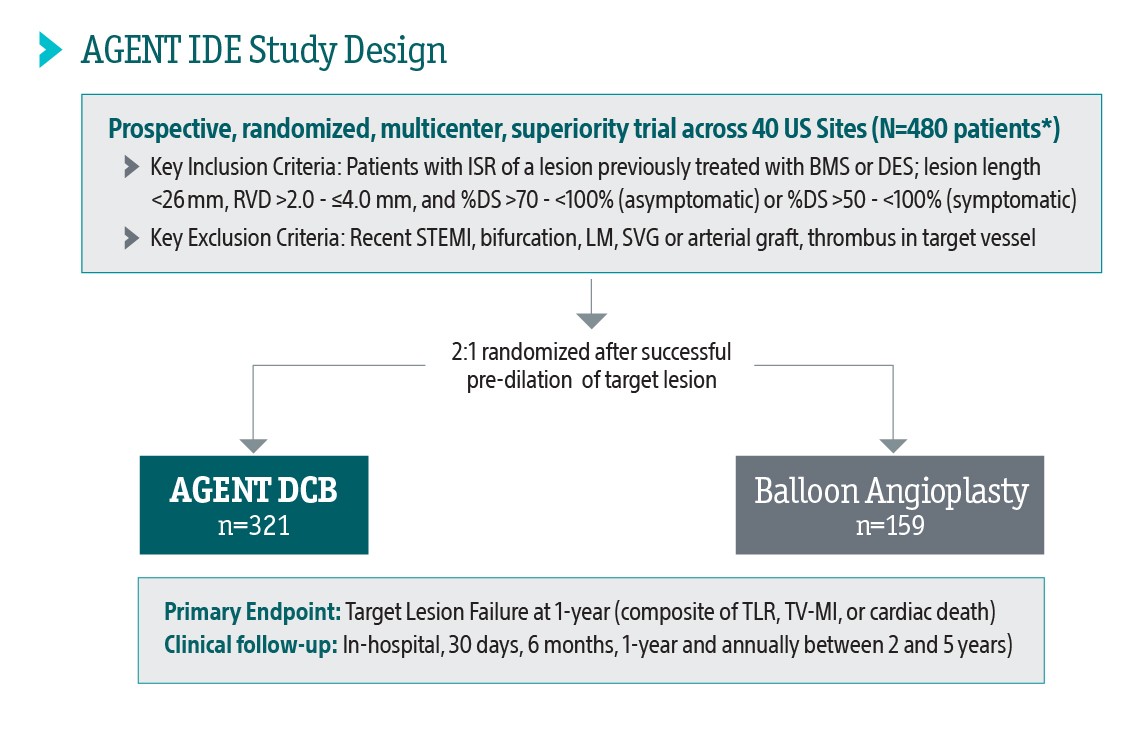
AGENT IDE Clinical Trial
Primary Endpoint²