Discussion:
The PREEMPT-HF study represents the largest study to date evaluating HeartLogic sensors in relationship to HF hospitalization and death. It is also the first large study to include ICD and CRT-D patients .
The results will inform the real-world performance of HeartLogic for several key outcomes that have not been evaluated previously.
Heart failure readmission:
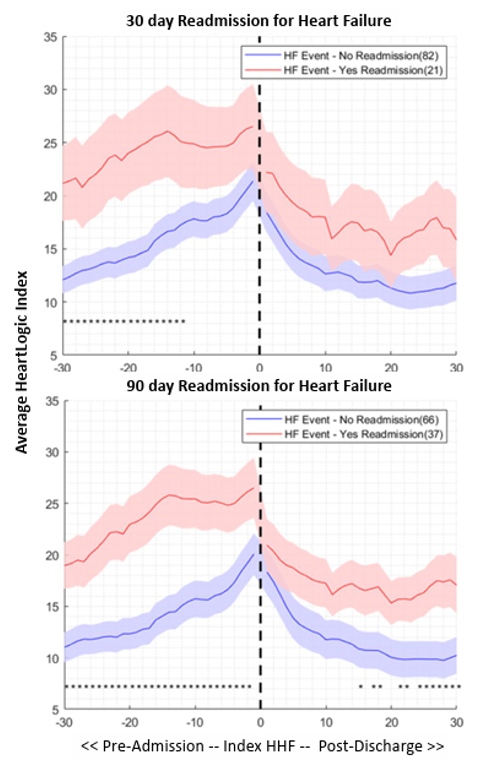
Hospitalizations due to worsening HF are associated with high post-acute re-hospitalization and mortality. Considering the large sample size, PREEMPT-HF is expected to have sufficient events that allow to evaluate sensor changes preceding hospital readmission. These data could contribute to a better understanding of discharge readiness and an improved comprehension of sensor changes within the 30-days subsequent to hospital discharge.
Arrhythmias:
It is well known that arrhythmias are more common as HF progresses. Considering the association between arrhythmias and worsening heart failure, it is crucial to better understand the relation of HF-related sensor data and the risk of tachyarrhythmias and device therapy.
Phenomapping:
Heart failure is composite of multiple heterogeneous entities and different classification of acute worsening heart failure has been proposed. Physiological measurements underlying clinical classifications exhibit significant variation. PREEMPT-HF offers a large data set for using phenomapping techniques to identify subgroups of HF patients.