TheraSphere™
Y90 Glass Microspheres
TheraSphereTM Y90 Microspheres take advantage of a tumor’s hypervascularity and prioritise microsphere flow to the tumor. Because TheraSphereTM has high activity per sphere, fewer microspheres are needed to achieve the desired dose. The minimally embolic nature of TheraSphereTM preserves the patient’s vasculature and allows for safe and effective delivery of radiation without the risk of stasis and reflux and spares healthy tissue.
Explore
Product Description
TheraSphereTM allows for personalization of treatment and greater flexibility by offering a multitude of standard and custom dose vial options to meet individual patient treatment goals1. TheraSphere has demonstrated treatment success in a range of HCC scenarios: across disease stages2,3 in patients with single4 or multifocal tumors5, portal vein thrombosis (PVT)6,7,8 and using segmental9 or lobar approaches10,11.
- IFU Section 1: Device Description (page 2)
- Salem R, Gabr A, et al. Institutional Decision to Adopt Y90 as Primary Treatment for HCC Informed by a 1,000-patient 15-year Experience. Hepatology 2017; 68(4):1429-1440
- Lam M, Garin E, et al. A global evaluation of advanced dosimetry in transarterial radioembolization of hepatocellular carcinoma with Yttrium-90: the TARGET study. Eur J Nuc Med Mol Imaging April 8, 2022
- Kim E, Sher A, et al. Radiation segmentectomy for curative intent of unresectable very early to early stage hepatocellular carcinoma (RASER): a single-centre, single-arm study. The Lancet Gastro Hep 2022; May 21st Online First
- Zu Q, Schenning R et al. Yttrium-90 Radioembolization for BCLC Stage C Hepatocellular Carcinoma Comparing Child-Pugh A Versus B7 Patients: Are the Outcomes Equivalent? Cardiovasc Intervent Radiol 2020;43:721-731
- Mazzaferro V, Sposito C, et al. Yttrium-90 radioembolization of intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57(5):1826-37.
- Abouchaleh N, Gabr A, et al. Y90 Radioembolization for Locally Advanced Hepatocellular Carcinoma with Portal Vein Thrombosis: Long-Term Outcomes in a 185-Patient Cohort. Journal of Nuclear Medicine 2018;59(7):1042-1048
- Garin E, Tselikas L et al. Personalized versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomized, multicentre, open-label phase 2 trial. The Lancet Gastro Hep 2021;6(1):17-29
- Salem R, Johnson G, et al. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021;74(5):2342-2352
- Lam M, Garin E, et al. A global evaluation of advanced dosimetry in transarterial radioembolization of hepatocellular carcinoma with Yttrium-90: the TARGET study. Eur J Nuc Med Mol Imaging April 8, 2022
- Garin E, Tselikas L et al. Personalized versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomized, multicentre, open-label phase 2 trial. The Lancet Gastro Hep 2021;6(1):17-29
Mechanism of Action
A healthy liver receives most of its blood flow (75%) from the portal vein 1. In HCC, tumor blood supply is almost exclusively from the hepatic artery (80-100%) 2. TheraSphereTM Y90 glass microspheres exploit tumor blood flow and are delivered to the tumor vasculature via catheterisation of the hepatic artery.
The glass microspheres penetrate the tumor arteriolar capillaries where they emit lethal beta radiation that is localised to the surrounding tumor tissue.3,4 The targeted distribution of microspheres provides high absorbed dose coverage to the tumor while sparing normal tissue.3,4,5,6
The high specific activity of TheraSphere glass microspheres means that fewer particles are administered to achieve the desired dose compared to resin Y-90 and 166Ho microspheres8,9. As a result, glass microspheres are minimally embolic without concern for vascular stasis10.
Since vessel patency is maintained, subsequent arterial therapies are possible, if needed.3,7
- Merkel C, Montagnese S, Amodio P. Functional Anatomy of Liver Circulation. Functional Molecular.
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolisation of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolisation brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68(1):13–23.
- Salem R, Thurston KG. Radioembolisation with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006;17:1251–78.
- Kulik M, Carr B, Mulcahy M, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47(1):71–81.
- Riaz A, Gates VL, Atassi B, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy off radioembolisation. Int J Radiat Oncol Biol Phys 2011;79(1):163–71.
- Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolisation for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57(5):1826–37.
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolisation results in longer time-to-progression and reduced toxicity compared with chemoembolisation in patients with hepatocellular carcinoma. Gastroenterology 2011;140(2):497–507.
- Westcott M, Coldwell D, et al. The Development, commercialization, and clinical context of yttrium-90 radiolabeled resin and glass microspheres. Advances in Radiation Oncology 2016;1, 351-364
- d'Abadie P, Hesse M, Louppe A, Lhommel R, Walrand S, Jamar F. Microspheres Used in Liver Radioembolization: From Conception to Clinical Effects. Molecules. 2021 Jun 29;26(13):3966. doi: 10.3390/molecules26133966. PMID: 34209590; PMCID: PMC8271370.
- Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol 2006;17:1251-78
Product Specifications and Sizes
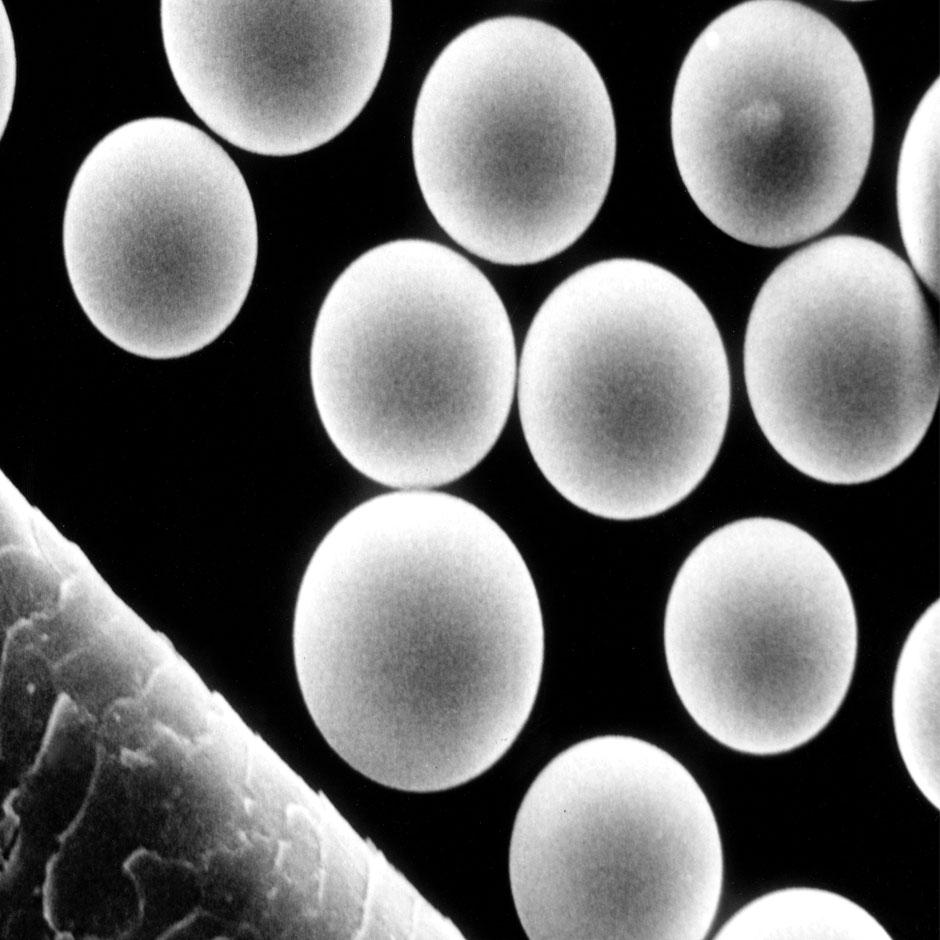
TheraSphereTM consists of Y90 glass microspheres used in radiation treatment for patients with hepatic malignancies.
TheraSphereTM Y90 glass microspheres
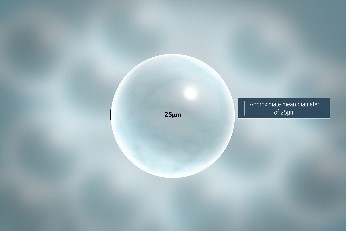
- Insoluble glass microspheres with a mean diameter of 15 to 35 μm
- Y90 is an integral constituent of the glass
Yttrium-90 (Y-90)
- Pure beta emitter
- Average energy of 0.9367 MeV
- Average tissue penetration range of 2.5 mm (max. 11 mm)
TheraSphere™ Order Form in PDF
Need a printed copy for your patient files? Please download the PDF on your computer, fill out the form and then submit directly to Customer Service or print out and fax your order.
Ordering Information

Latest evidence with TheraSphere™
Simplicit90Y™