Clinical outcomes you can trust
Recent clinical data continues to reinforce AGENT™ Drug-Coated Balloon (DCB) as a proven solution for treating coronary artery disease. AGENT™ DCB has been used in more than 275.000 patients worldwide and studied in more than 16.000 patients across complex in-stent restenosis (ISR) and de novo populations.¹
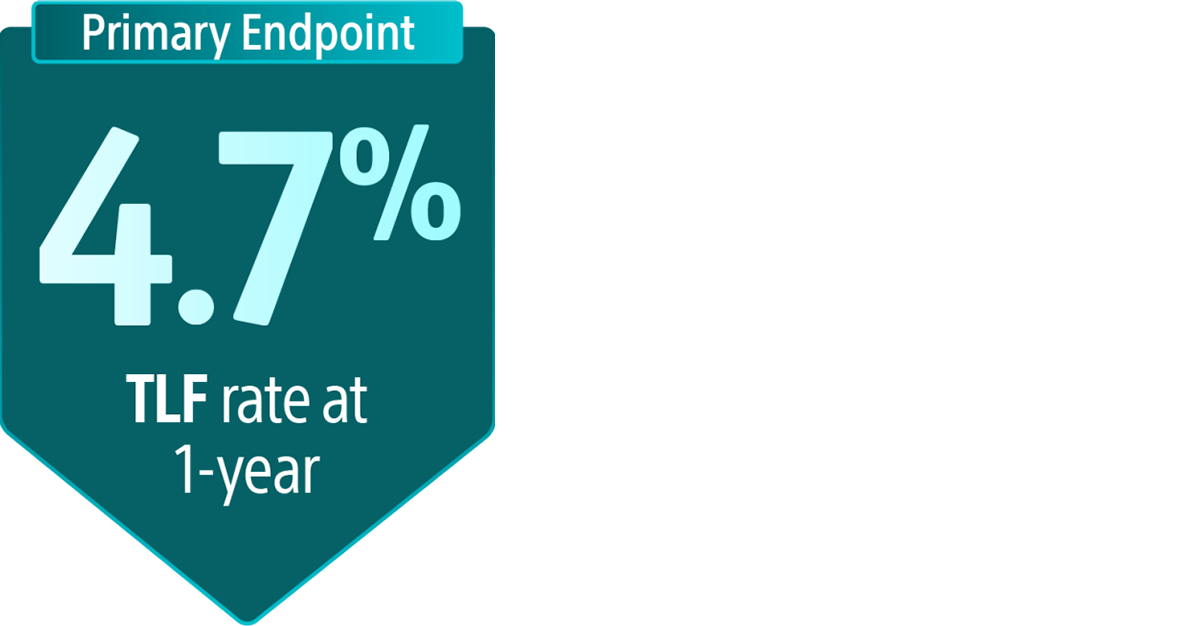
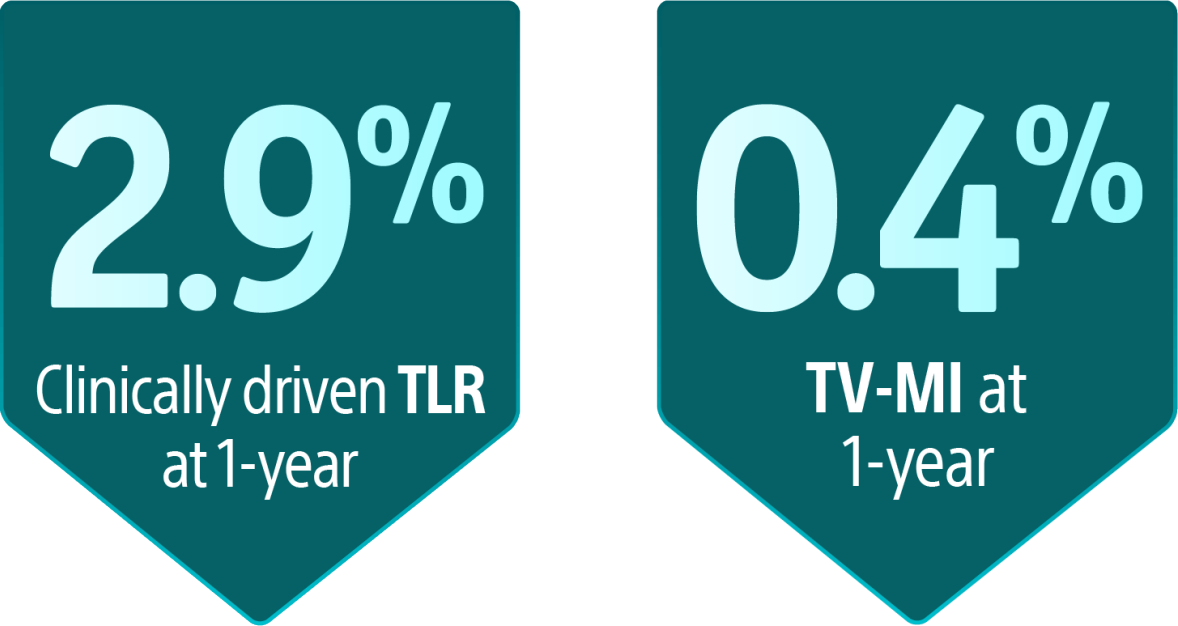
*Data shown may include results from studies using the product outside its approved labelling. These findings are presented for scientific discussion only. Please refer to the Instructions for Use (IFU) or product labelling for approved indications and usage.




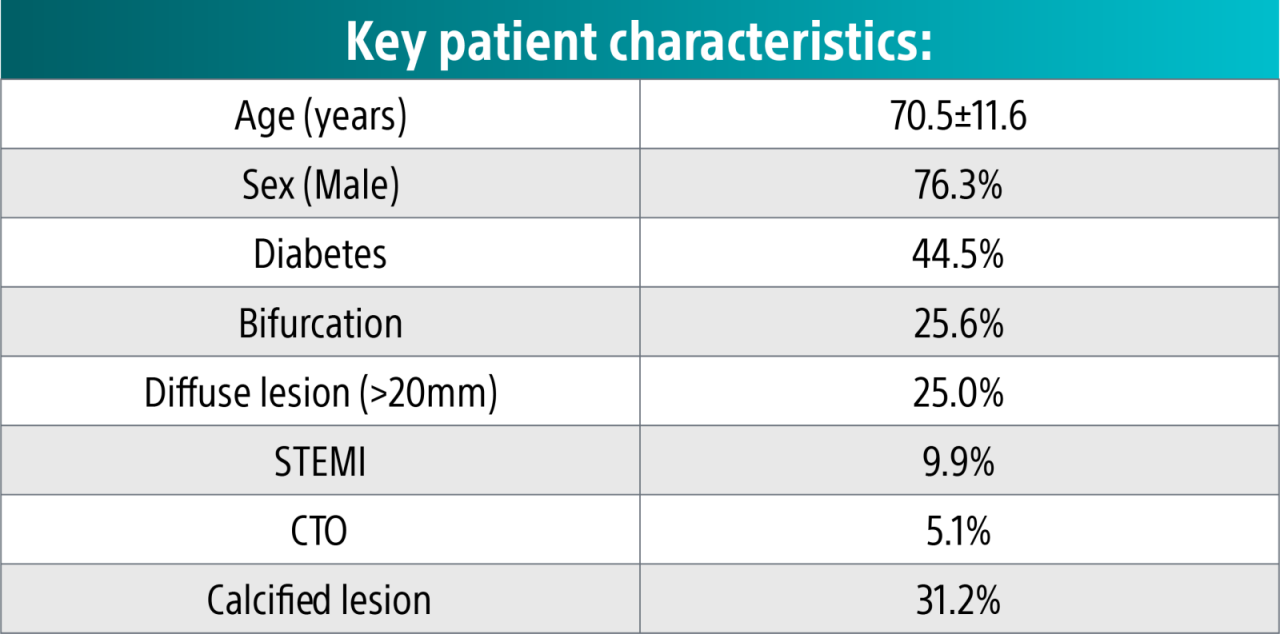
In-hospital safety outcomes for ISR PCIs **
Myocardial infarction (MI)

Bleeding

All cause mortality

All cause mortality

Myocardial infarction (MI)

Bleeding

Heart failure

Ischemic stroke

This study showed approximately 17.5% of patients undergoing ISR PCI are now treated with a DCB. It also found DCB is more often used with intravascular imaging and adjunctive prep devices.⁵
*27.2% of AGENTᵀᴹ ISR use (3,459/12,729 procedures) through June 2025 was for an off-label indication. These findings are presented for scientific discussion only. Please refer to the Instructions for Use (IFU) or product labelling for approved indications and usage.
**AGENTᵀᴹ DCB n=9,269 and DES n=65,890.
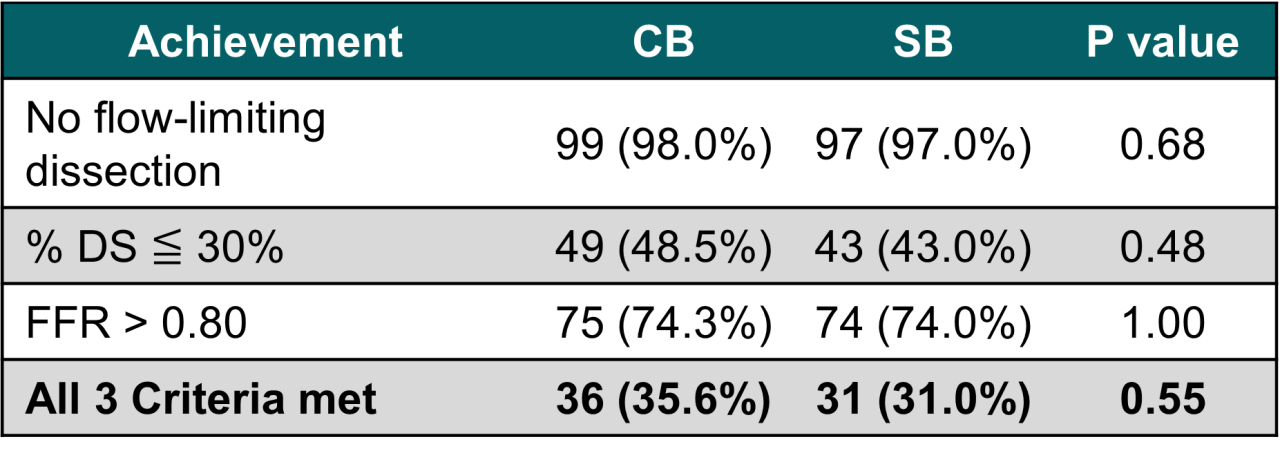
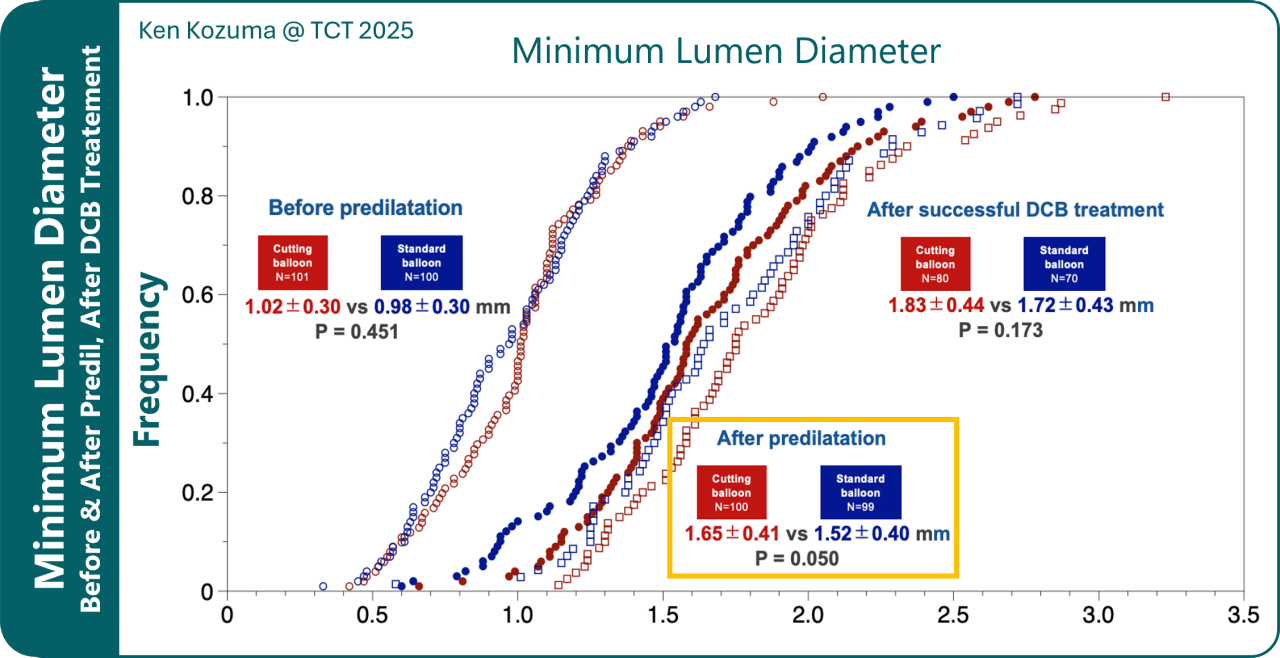
Cutting Balloon led to significantly better pre-DCB preparation based on Minimum Lumen Diameter (MLD) suggesting the possibility of better longterm results and may lead to improved drug delivery and long-term outcomes in DCB procedures.
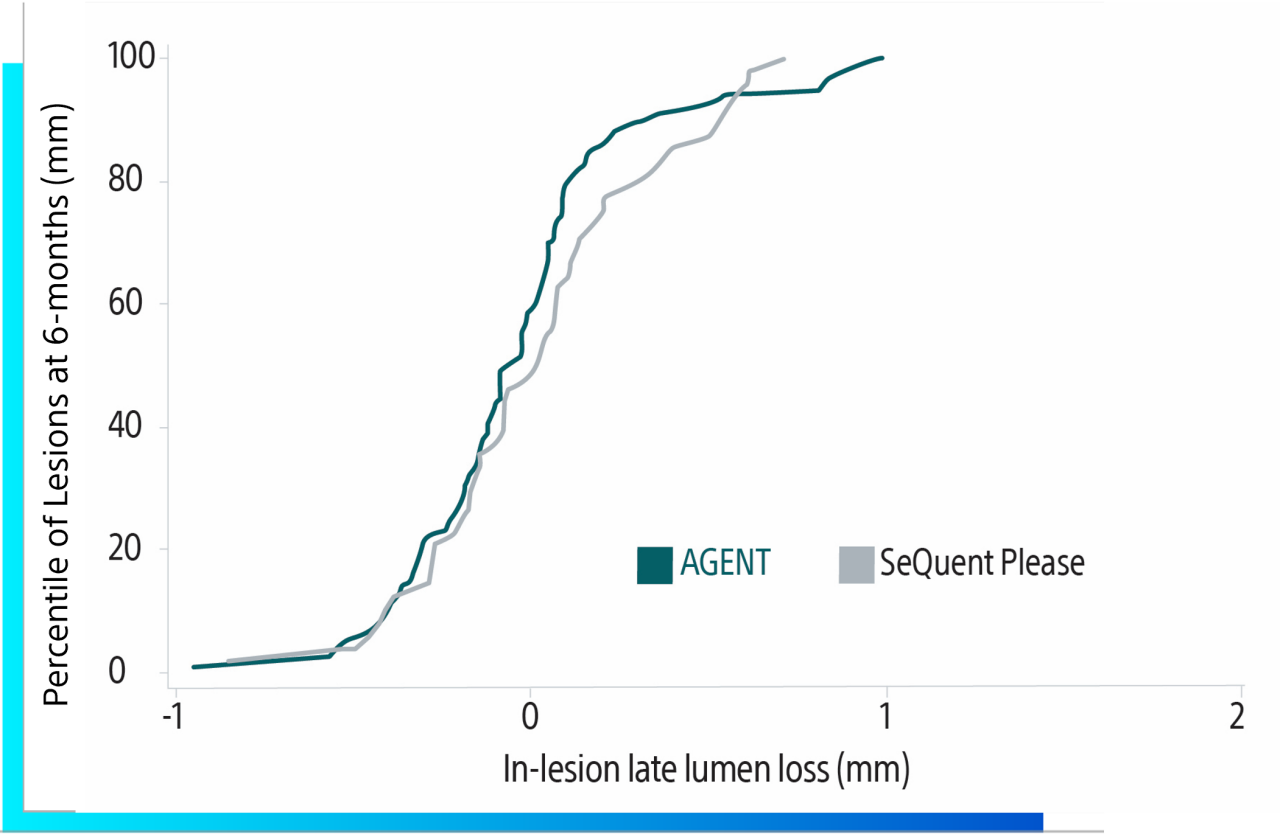
Further findings about using HD-IVUS in correlations with 12-month composite endpoints and 9-month LLE (after DCB treatment) are not yet disclosed. These results will support previous findings in a rabbit model for improved drug delivery⁵.
The NATURE trial demonstrates that WOLVERINEᵀᴹ Cutting Balloon, through its mechanism of action - creating controlled microincisions, providing anchoring stability, and amplifying dilatation force - enables safe and predictable plaque modification.
1. Data on file at BSC as of October 2025.
2. Nakamura, et al. Paclitaxel Drug-coated Balloon Angioplasty for De Novo Coronary Lesions in an Expanded Real World Clinical Setting: The Multicenter ALLIANCE Registry. medRxiv. 3 December 2025. Pre-print before peer-reviewed
publishing. doi: https://doi.org/10.64898/2025.11.30.25341329
3. Data presented at TCT 2025 by Dr. Masato Nakamura. Results include over 1,300 Japanese patients treated with AGENT DCB (73.3% of the 1,800 PTX DCB patients).
4. Nakamura, M, et all, Drug-coated balloon for the treatment of small vessel coronary artery disease, Circulation Japan 2022 AGENT IDE Clinical Trial data presented at CRT 2024 by Dr. Robert Yeh.
5. Data presented at TCT 2025 by Dr. Christina Lalani.
6. Ken Kozuma @ TCT 2025
7. Shiozaki, M., et al. Catheterization and Cardiovascular Interventions (2025).
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.