1. Surapaneni, M., et al. "Designing Paclitaxel drug delivery systems aimed at improved patient outcomes: current status and challenges." International Scholarly Research Notices (2012).
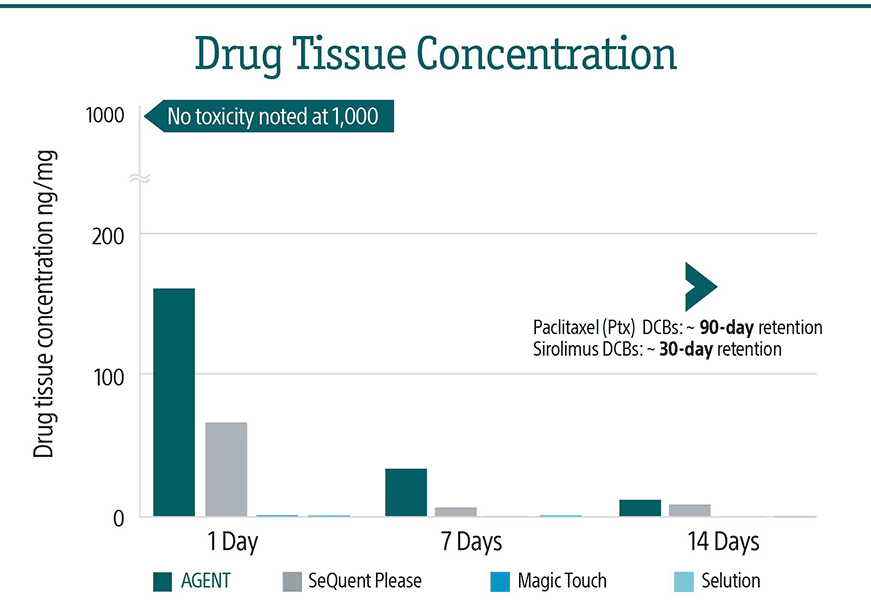
2. Tzafriri A, et al. Journal of Controlled Release. 2019; 310:94–102. Taking paclitaxel coated balloons to a higher level: Predicting coating dissolution kinetics, tissue retention and dosing dynamics
3. BSC Internal Test. Results of internal bench studies are not representative of clinical performance. Clinical results may vary.
4. Speck, U,. et al., Do Pharmacokinetics Explain Persistent Restenosis Inhibition by a Single Dose of Paclitaxel?, Circ Cardiov Interv, 2012
5. Loh, JP., et al., The current status of drug-coated balloons in percutaneous coronary and peripheral interventions, EuroIntervention 2013
6. Tzafriri AR, et al., Taking paclitaxel coated balloons to a higher level: Predicting coating dissolution kinetics, tissue retention and dosing dynamics. J Control Release. 2019 Sep
7. Pre-clinical pharmacokinetic studies performed using the same FDA recommended and accepted porcine coronary de nevo vessel test methodBSC conducted AGENT, SeQuent Please and Selution. Magic touch published data, TCT 2020.
8. Granada JF, et al. Mechanisms of tissue uptake and retention of paclitaxel-coated balloons: impact on neointimal proliferation and healing. Open Heart 2014
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries.
This material not intended for use in France.