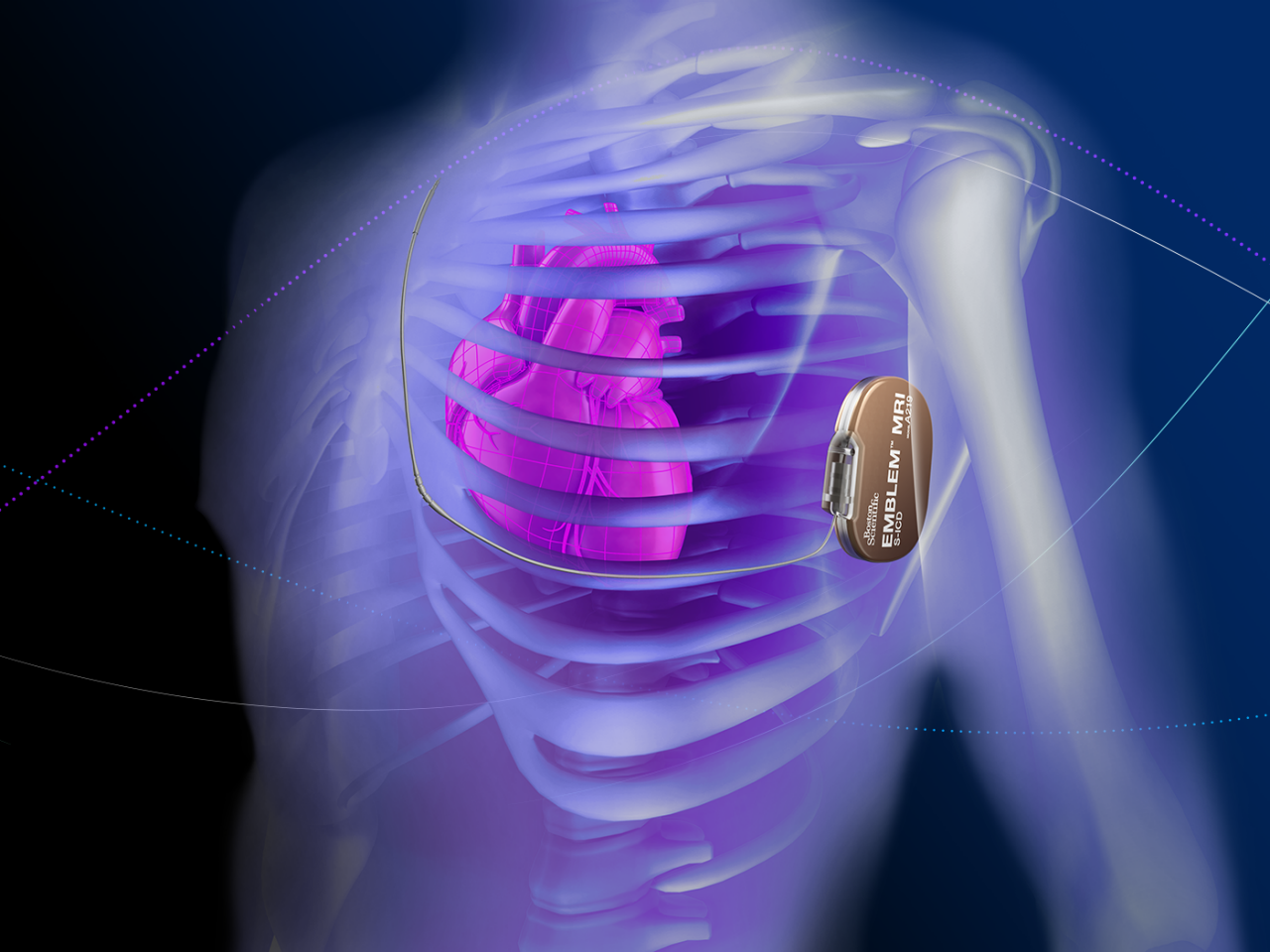
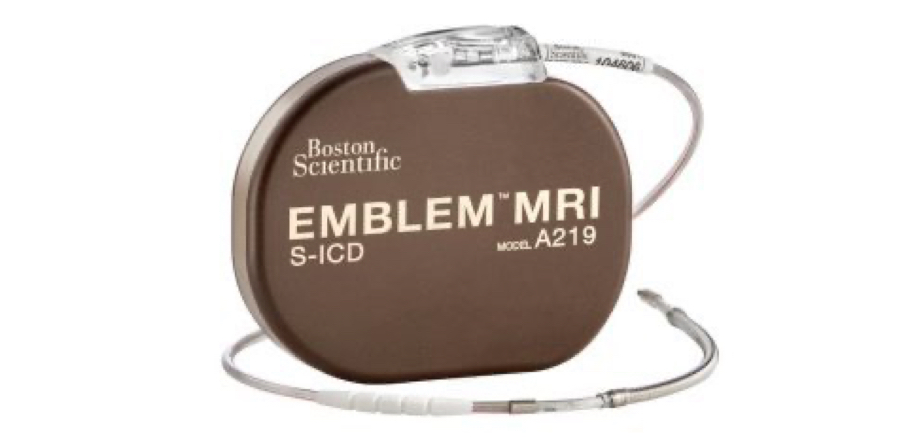
*EMPOWER™ component pending CE Mark. Not available for sale.
References:
1. Gasparini, M, Lunati, MG, Proclemer, A, et al. Long Detection Programming in Single-Chamber Defibrillators Reduces Unnecessary Therapies and Mortality. JACC Clin Electrophysiol. 2017;3(11):1275–1282.
2. Data on VR devices from LATITUDE Boston Scientific data on file.
3. Botto GL, Forleo GB, Capucci A, , et al. The Italian subcutaneous implantable cardioverter-defibrillator survey: S-ICD, why not? Europace. 2017;19(11):1826–1832.
4. Boston Scientific. Data on file.
ATP, antitachycardia pacing; CRM, cardiac rhythm management; ICD, implantable cardioverter defibrillator; LP, leadless pacemaker; MVT, monomorphic ventricular tachycardia; S-ICD, subcutaneous ICD.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device. or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.