SYNERGY™ & SYNERGY MEGATRON™
EES PtCr Coronary Stent System
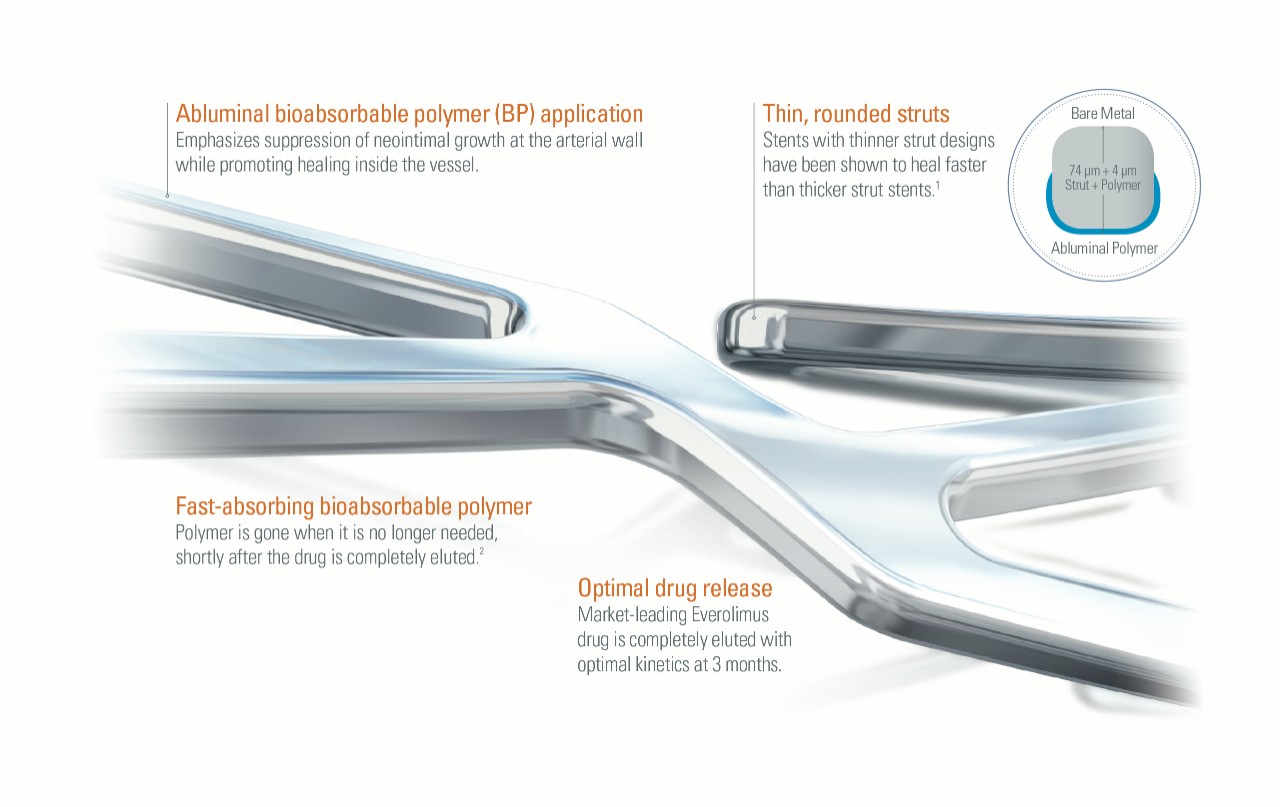
The SYNERGY BP Stent works synergistically, emphasizing the suppression of neointimal growth at the arterial wall while promoting healing inside the vessel. It features the Synchrony™ Bioabsorbable Polymer Coating, which is applied only to the abluminal side of the stent to promote rapid endothelialization.1,2
Explore
Freedom from Long-Term Polymer Exposure
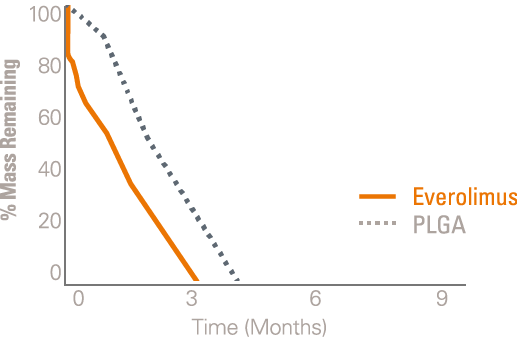
The polymer on the SYNERGY BP Stent is absorbed when it’s no longer needed, shortly after the drug is completely eluted at three months, providing early healing and freedom from long-term polymer exposure.1
It was designed to address the challenges associated with permanent polymer stents such as inflammation, neoatherosclerosis and late stent thrombosis.
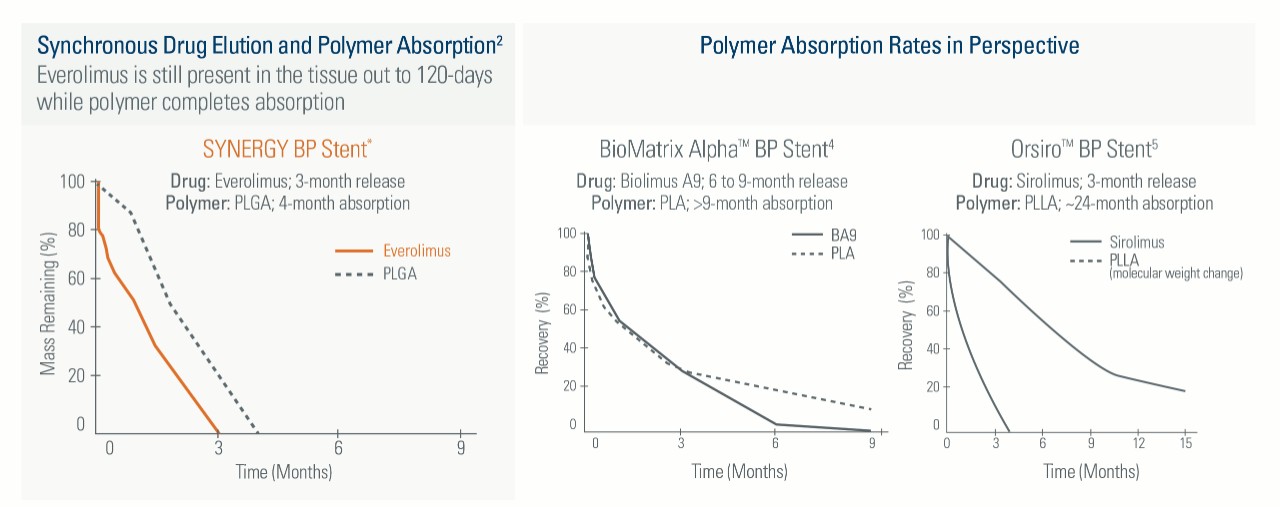
Everolimus is still present in the tissue out to 120-days while polymer completes absorption
1. Wilson GJ et al, Catheter Cardiovasc Interv. 2015.
2. Eppihimer M, PhD. Impact of Polymer Type and Location on Stent Thrombogenicity and Endothelial Cell Coverage. EuroPCR 2014.
3. Soucy N, Feygin J et al, EuroIntervention. 2010 Nov;6(5):630-7.
4. Presented by J. M. de la Torre, MD at EuroPCR 2014.
5. Presentation by Kazuyuki Yahagi, MD; Michael Joner, MD; Renu Virmani, MD at TCT 2014.
6. Adapted from presentations by Juan Granada, MD at TCT 2014 (and PCR 2014). Evaluated the PtCr Bare-Metal Stent. Preclinical: Diseased Porcine Model.
Early Healing
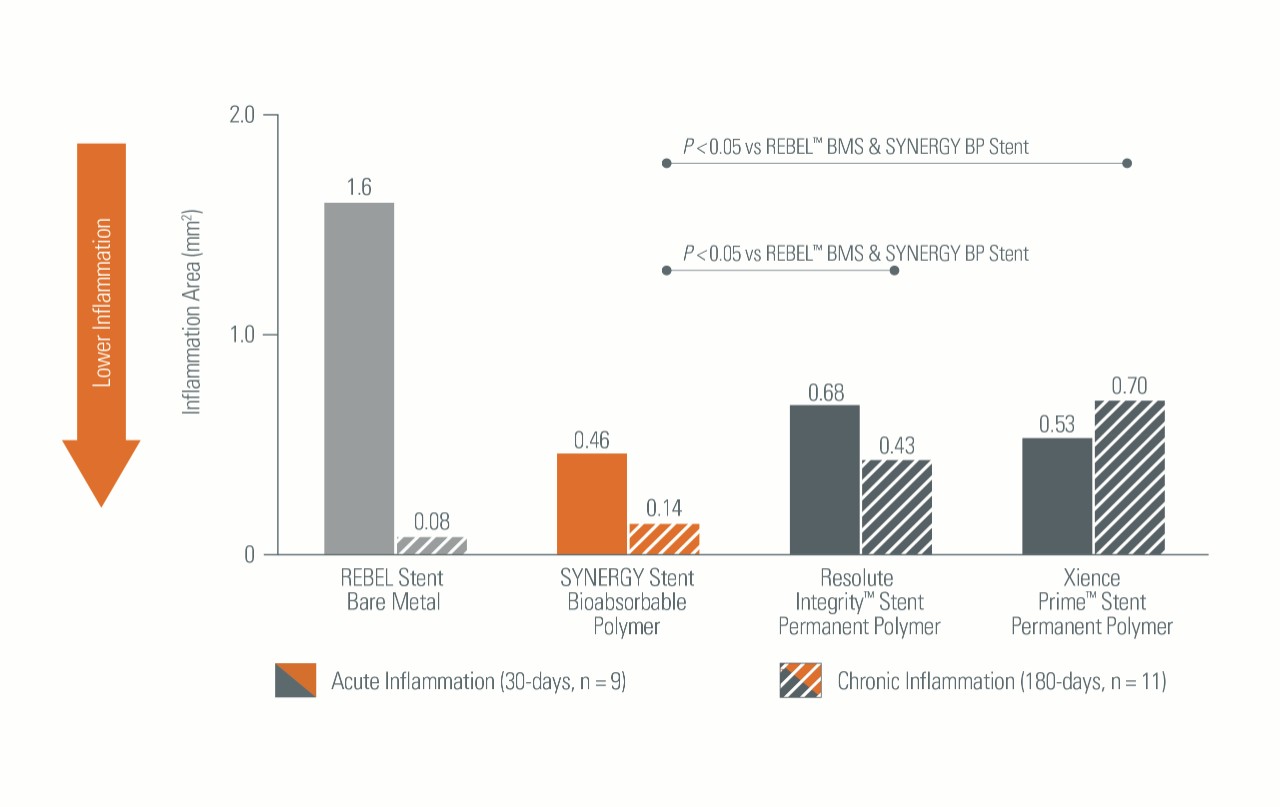
Lower Inflammation Rates3
In a preclinical model, SYNERGYTM BP Stent demonstrated:
- Significantly reduced Early Inflammation versus Bare Metal Stents (BMS)
- Significantly reduced Late Inflammation versus Permanent Polymer (PP) Stents
Not All Bioabsorbable Polymer Stents are Created Equal
Optimal Healing Shown Through Strut Coverage and Angioscopy
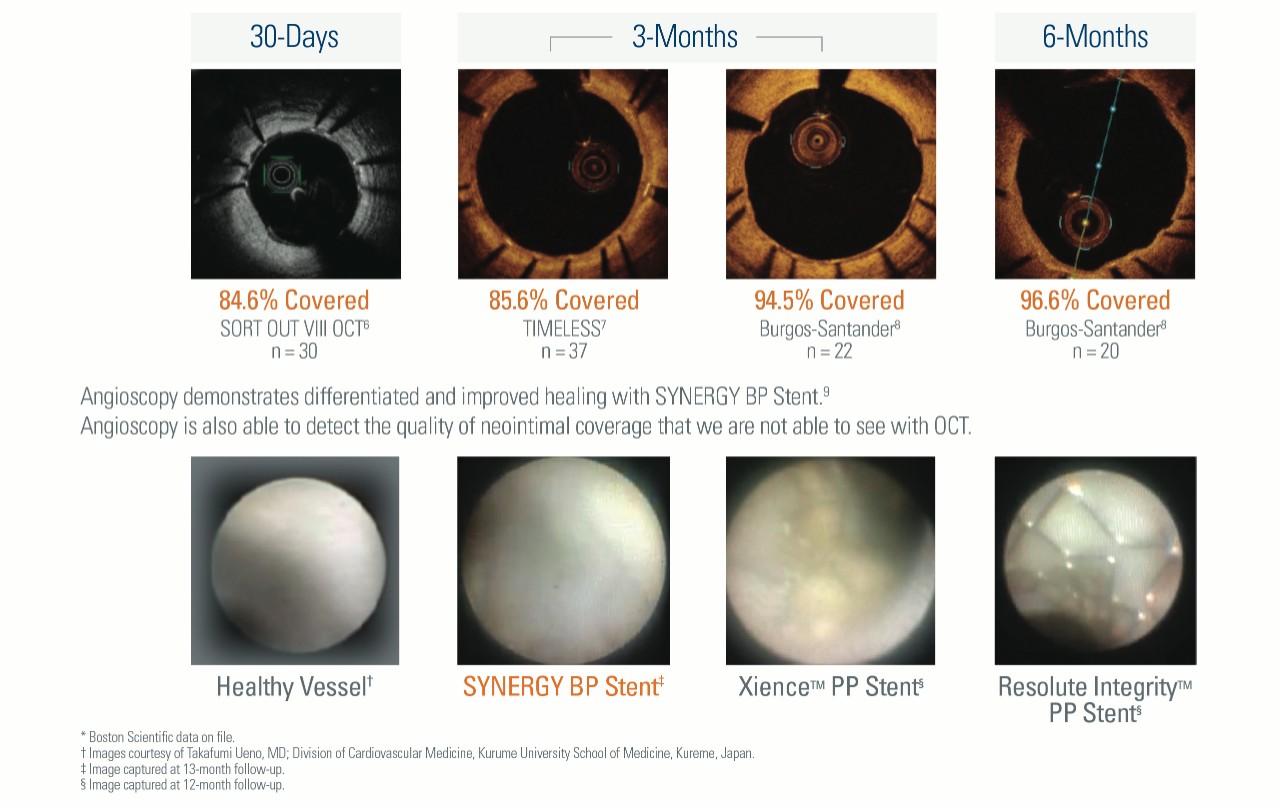
2. Wilson GJ, et al. Catheter Cardiovasc Interv.
3. Adapted from presentations by Juan Granada, MD at TCT 2014 (and PCR 2014). Evaluated the PtCr Bare-Metal Stent. Preclinical: Diseased Porcine Model.
4. Garg, S, J Am Coll Cardiol. 2010;56 (10s1):S43-S78. doi:10.1016.
5. Presented by Stephan Windecker, MD, TCT2012.
6. Adapted from presentation by Ida Riise Balleby, MD at ACC 2016.
7. Adapted from presentation by Juan Granada, MD, at CRT 2015.
8. Adapted from presentation by JM de la Torre, MD at PCR 2015.
9. Nakayoshi T, Ueno T, Sasaki K, et al. Differential angioscopic findings of neointimal coverage among first-, second-, and next generation drug eluting stents. Int J Cardiol. 2016;223:450-451. Images courtesy of Professor Ueno.