SpaceOAR Vue™ Hydrogel
Radiopaque Perirectal Spacer for Radiation Therapy

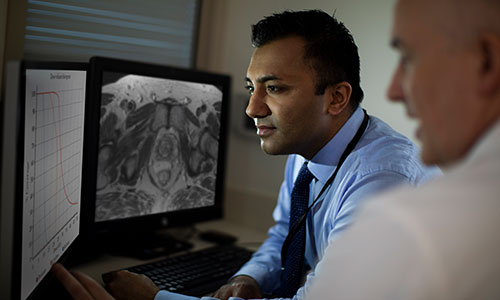
Enhanced Visibility
SpaceOAR Vue Hydrogel delivers similar clinical benefits as SpaceOAR Hydrogel, plus enhanced CT visibility3
Access SpaceOAR Hydrogel Evidence

Minimal Risk
Iodinated contrast media reactions occur in less than 1% of the population, with few or no symptoms.1,2
Download The Infographics >

Clinical Evidence
SpaceOAR Hydrogel when compared to control demonstrates:4
-66% less v70 rectal irradiation
-77% reduction in the risk of rectal
toxicity (grade>2) in late follow-up
Access Meta Analysis
- Mammarappallil JG, Hiatt KD, Vincent W, et al. How accurate is the label “allergic to iodinated contrast agents”? Acta Radiol. 2016 Jan;57(1):47-50.
- Cha MJ, Kang DY, Lee W, et al. Hypersensitivity reactions to iodinated contrast media: A multicenter study of 196 081 patients. Radiology. 2019 Oct;293(1):117-24
- Data on file with Boston Scientific
- Larry E. Miller, PhD, PStat; Jason A. Efstathiou, MD, DPhil; Samir K. Bhattacharyya, PhD; Heather A. Payne, FRCR, FRCP; Emily Woodward, MSc; Michael Pinkawa, MD, PhD; Association of the Placement of a Perirectal Hydrogel Spacer With the Clinical Outcomes of Men Receiving Radiotherapy for Prostate Cancer - A Systematic Review and Meta-analysis. JAMA Network Open. 2020;3(6):e208221. doi:10.1001/jamanetworkopen.2020.8221. June 17, 2020.
*Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.2.2020 © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed July 8, 2020. To view the most recent and complete version of the guideline, go to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Information for use only in countries with applicable health authority registrations. This material not intended for use in France.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. Please check availability with your local sales representative or customer service.
SpaceOAR and SpaceOAR Vue Hydrogels are intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer and in creating this space it is the intent of SpaceOAR and SpaceOAR Vue Hydrogels to reduce the radiation dose delivered to the anterior rectum.
As with any medical treatment, there are some risks involved with the use of SpaceOAR and SpaceOAR Vue Hydrogels. Potential complications associated with SpaceOAR and SpaceOAR Vue Hydrogels include, but are not limited to: pain associated with SpaceOAR and SpaceOAR Vue Hydrogels injection; pain or discomfort associated with SpaceOAR and SpaceOAR Vue Hydrogels; needle penetration of the bladder, prostate, rectal wall, rectum or urethra; injection of SpaceOAR and SpaceOAR Vue Hydrogels into the bladder, prostate, rectal wall, rectum or urethra; local inflammatory reactions; infection; injection of air, fluid or SpaceOAR and SpaceOAR Vue Hydrogels intravascularly; urinary retention; rectal mucosal damage, ulcers, necrosis; bleeding; and rectal urgency.
All images are the property of Boston Scientific. All trademarks are the property of their respective owners.















