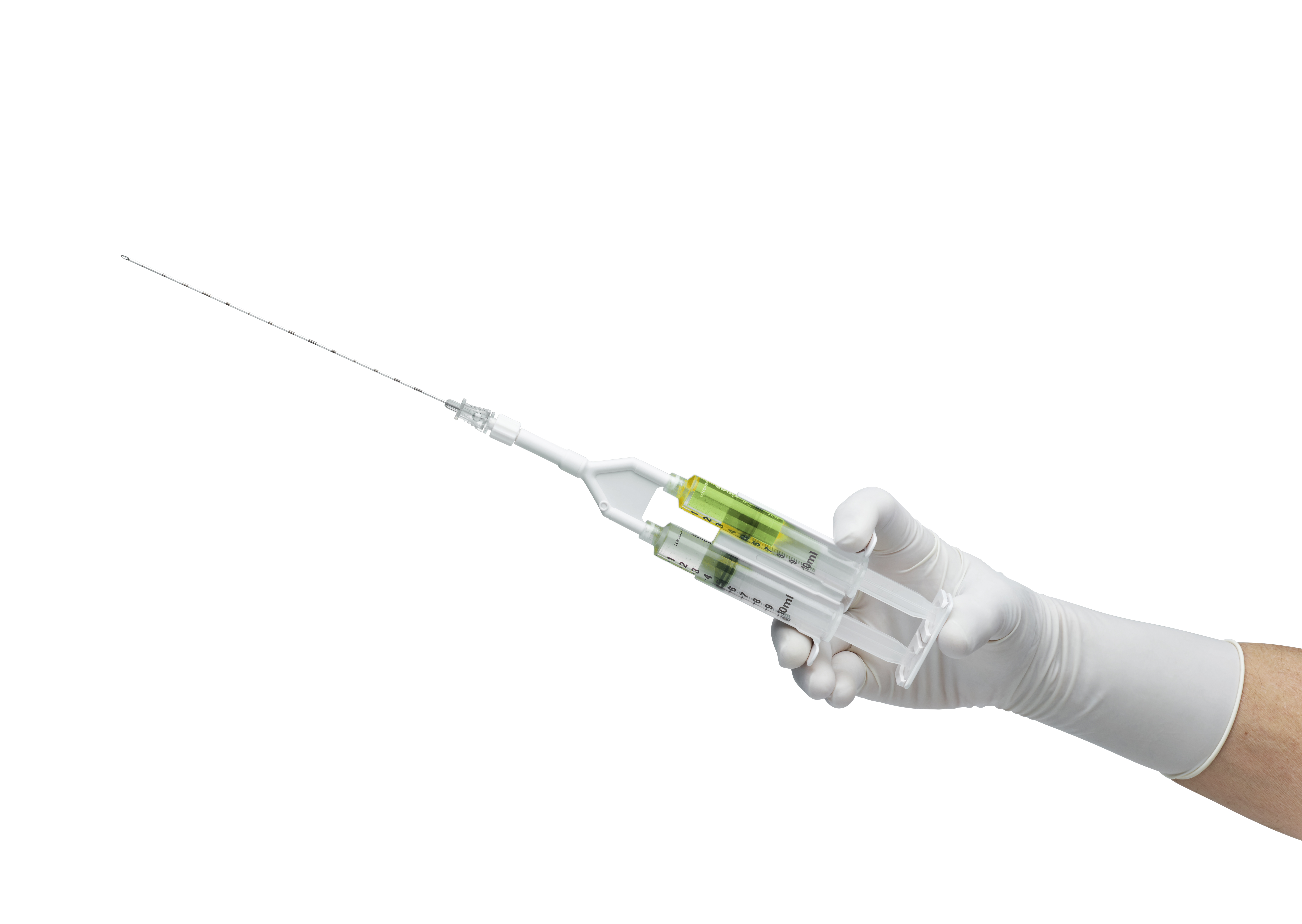
SpaceOAR Vue™ Hydrogel
Radiopaque Perirectal Spacer for Radiation Therapy
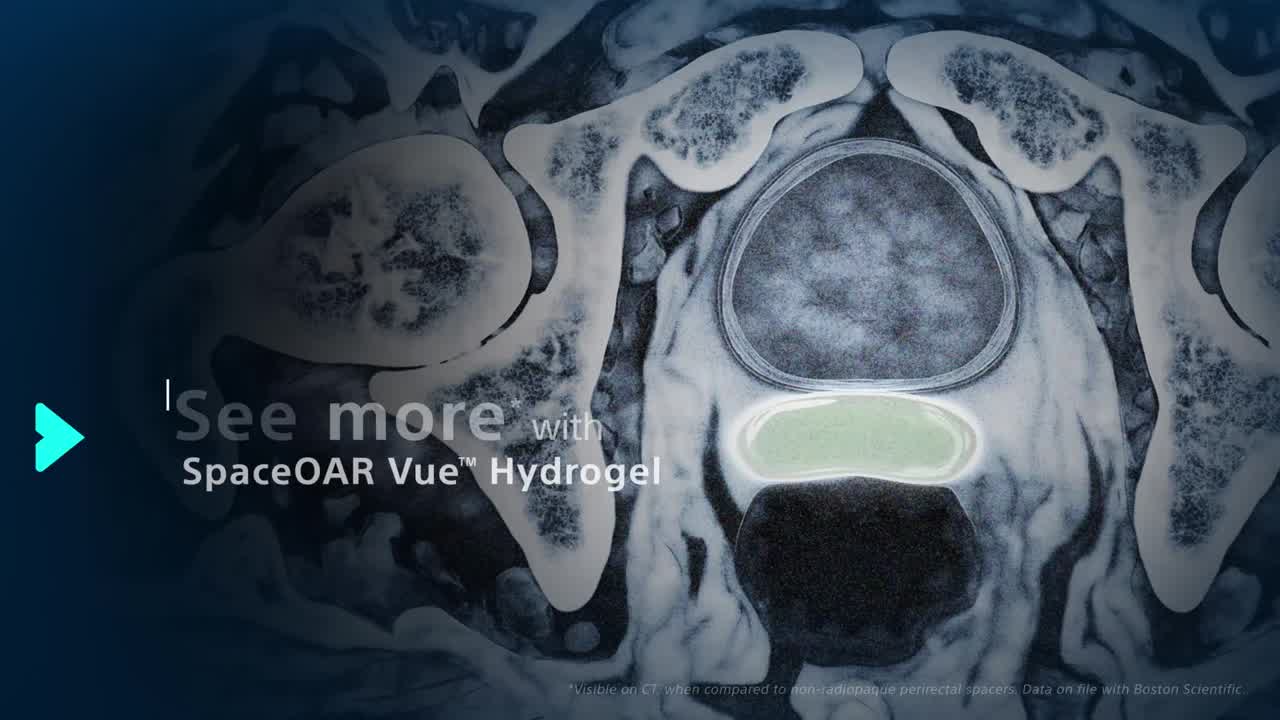
SpaceOAR Vue Hydrogel is a new generation hydrogel spacer that offers enhanced visibility via CT scan, designed to help physicians improve contouring accuracy and consistently position patients receiving prostate cancer radiation, as compared to SpaceOAR™ Hydrogel.1
Key Resources

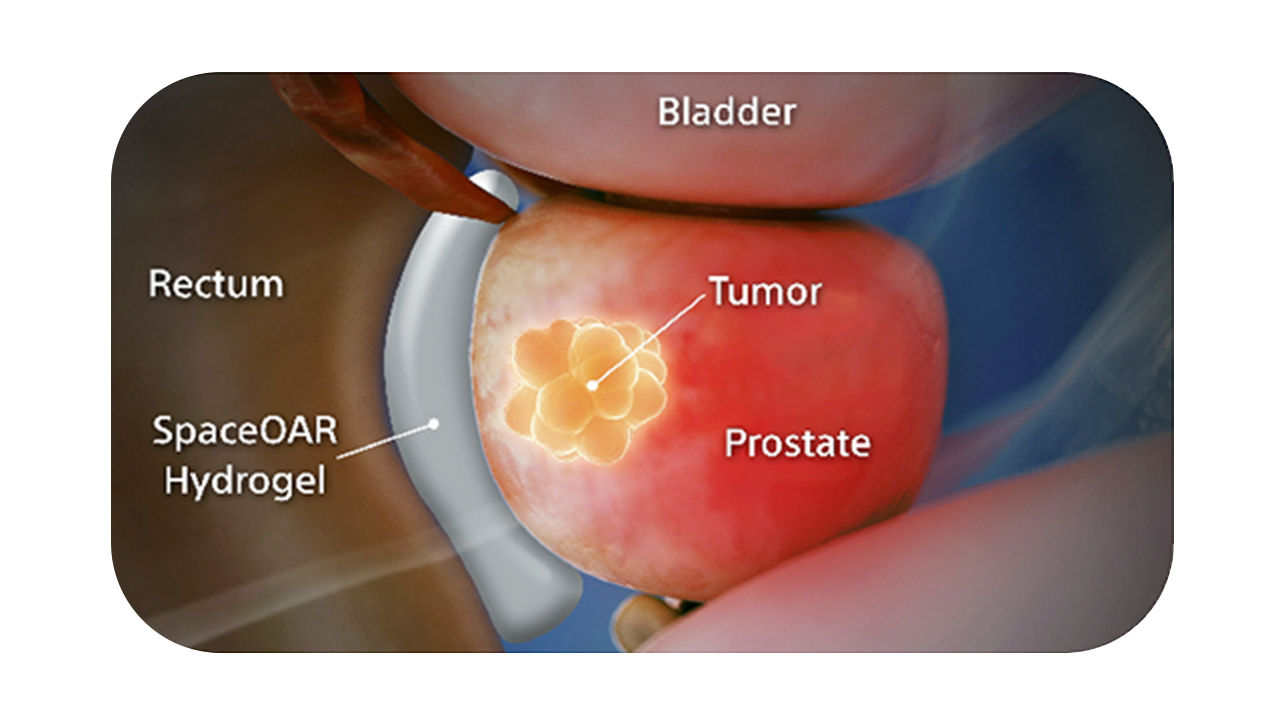
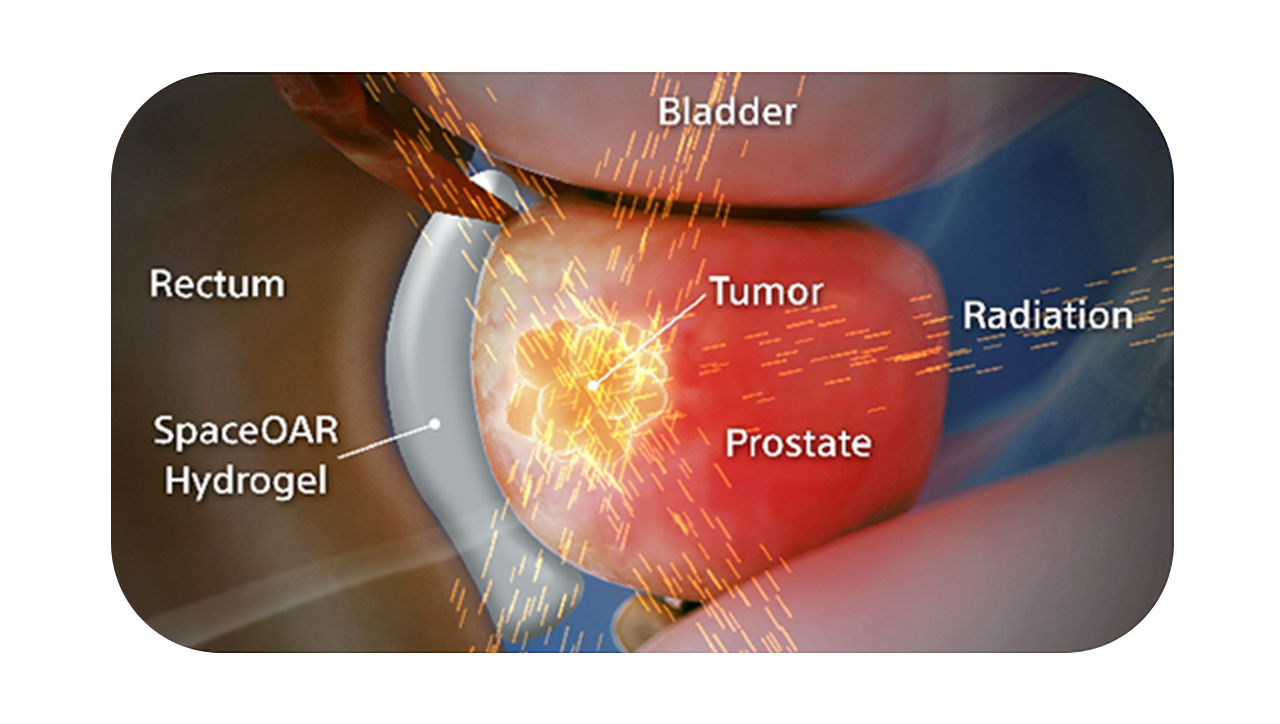
How it works
Why choose SpaceOAR Vue Hydrogel
The radiopacity is designed to improve the contouring accuracy during treatment plan creation, when compared to SpaceOAR Hydrogel.1
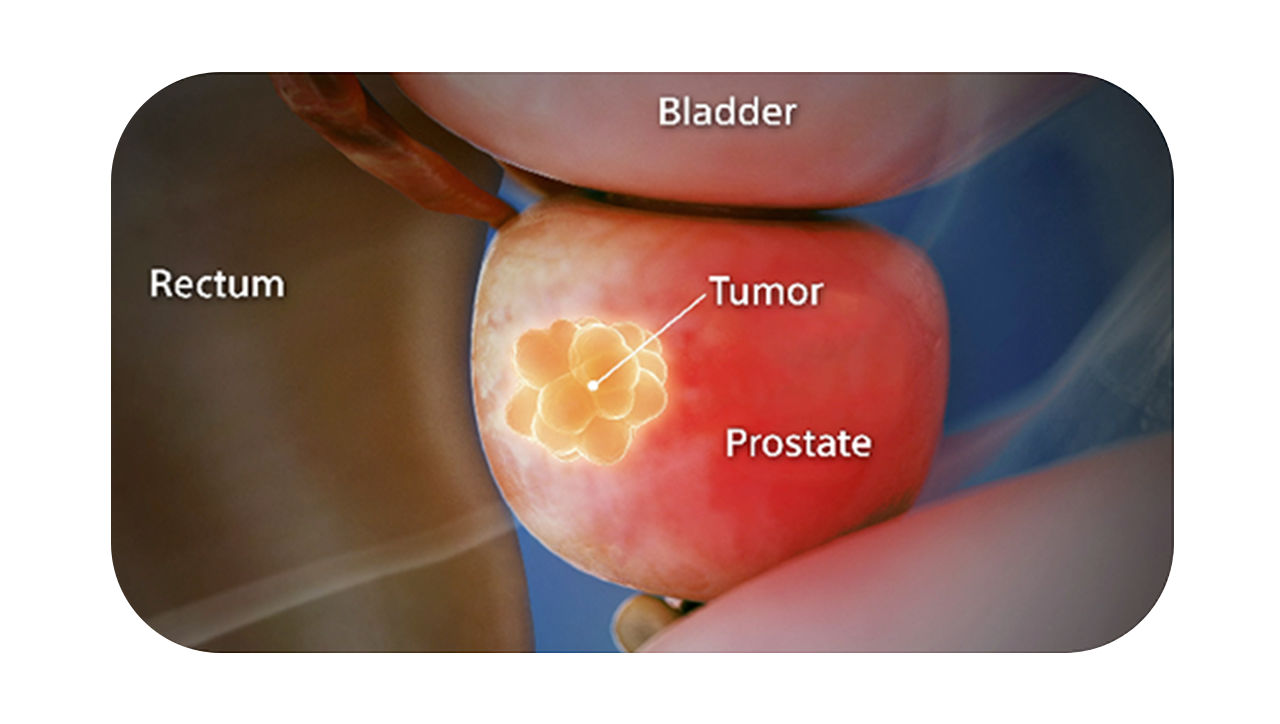
Benefits
Optimize dosing plan
Visibility of the gel on a CT scan designed to help to reduce under- or over-contouring of the prostate and rectum for improved accuracy, as compared to SpaceOAR Hydrogel, and helps optimize treatment planning.1,2
Streamline workflow
Treatment planning can be done using CT only, reducing the need to acquire, reference and fuse MR and CT images to aid in visualization of the target region enabling appropriate patient positioning for radiation treatment using kV cone-beam CT.1
Treat more patients
The radiopacity may provide a suitable imaging option to MRI for patients with implanted metallic devices.1
Ordering Information
| Product | Ordering Information |
| SpaceOAR Vue | SV-1010 |
Interested in learning more about SpaceOAR Vue Hydrogel?

Refrences:
1. Data on file with Boston Scientific
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Information for use only in countries with applicable health authority registrations. This material not intended for use in France.
Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. Please check availability with your local sales representative or customer service