Detachable Coils Portolio
Solutions for FRAMING, FILLING and FINISHING in Peripheral Embolization
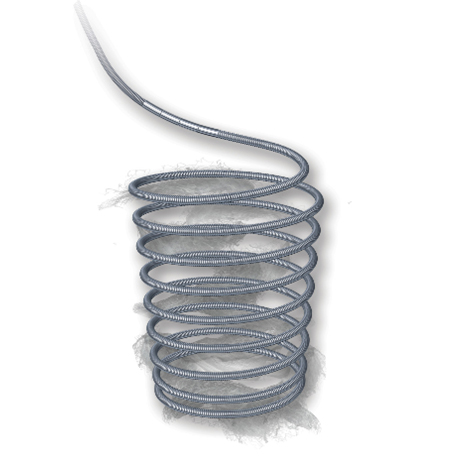
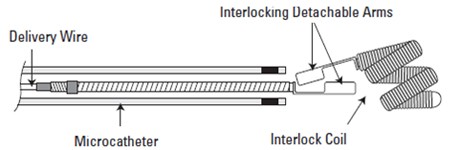
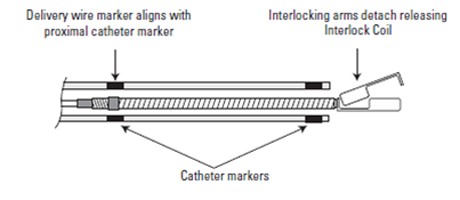
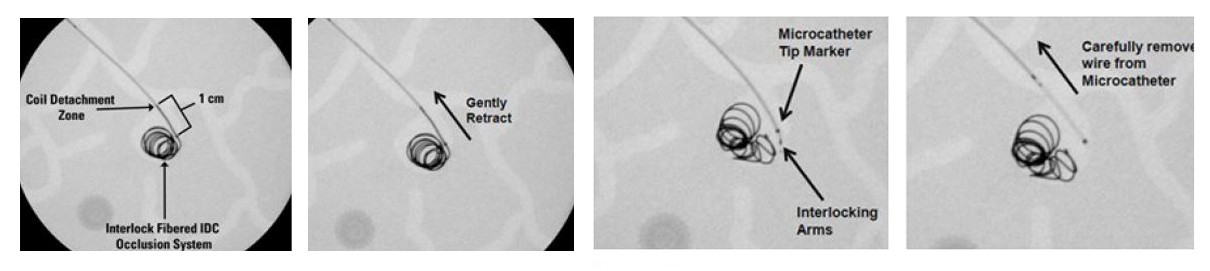
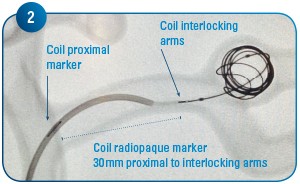
Associates Precision & Control of a detachable coil to the powerful Thrombogenicity of a fibered coil configuration. The Interlocking arm is designed to allow the coil to be advanced and retracted before final placement. Synthetic streamlined oriented & dense fibers onto the platinum coil foster stasis to help reach occlusion. Available in a broad range of product sizes, Interlock™ Fibered occlusion system offers precise treatment and clinical flexibility.
Key Resources
Product details
Shapes
Fibered for Thrombogenicity Power
- Every Coil contains multiple dense networks of Dacron® Fibers, each designed to catch blood cells and propagate the formation of thrombus.
- A close-up image of Dacron Fibers (left) shows that the structure of the fiber strands are robust, yet porous, allowing for rapid thrombosis.
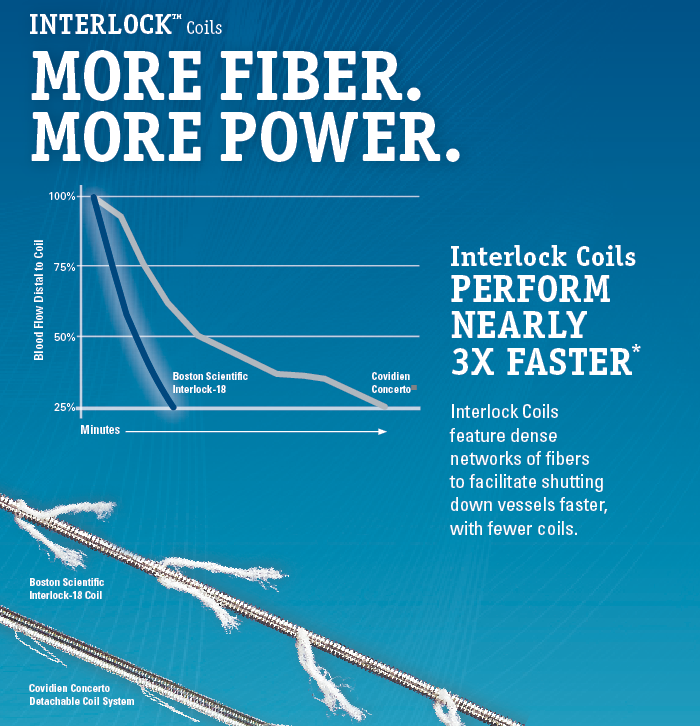
Independent Thrombogenicity Data
Interlock-18 fibered coils perform faster:
- It reaches occlusion over 2 times faster than Covidien Concerto*.
- Penumbra Ruby™ Coils take over 50% longer to reach same level of occlusion*.
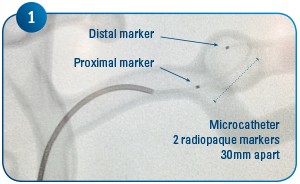
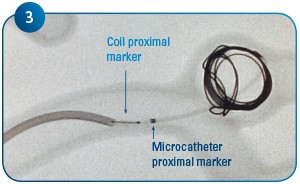
Catheter Compatibility
Coil delivery
Ordering information
Standard Length 2D Configurations
| UPN | Description | Diameter (mm) | length (mm) | Shape |
|---|---|---|---|---|
| M001361480 | Interlock - 18 Coil | 2 | 40 | 2D |
| M001361490 | Interlock - 18 Coil | 2 | 60 | 2D |
| M001361500 | Interlock - 18 Coil | 3 | 60 | 2D |
| M001361510 | Interlock - 18 Coil | 3 | 120 | 2D |
| M001361520 | Interlock - 18 Coil | 4 | 80 | 2D |
| M001361530 | Interlock - 18 Coil | 4 | 150 | 2D |
| M001361540 | Interlock - 18 Coil | 5 | 80 | 2D |
| M001361550 | Interlock - 18 Coil | 5 | 150 | 2D |
| M001361560 | Interlock - 18 Coil | 6 | 100 | 2D |
| M001361570 | Interlock - 18 Coil | 6 | 200 | 2D |
| M001361580 | Interlock - 18 Coil | 8 | 200 | 2D |
| M001361590 | Interlock - 18 Coil | 10 | 200 | 2D |
| M001361600 | Interlock - 18 Coil | 10 | 300 | 2D |
| M001361610 | Interlock - 18 Coil | 12 | 200 | 2D |
| M001361620 | Interlock - 18 Coil | 12 | 300 | 2D |
| M001361630 | Interlock - 18 Coil | 14 | 200 | 2D |
| M001361640 | Interlock - 18 Coil | 14 | 300 | 2D |
Long Length 2D Configurations
| UPN | Description | Diameter (mm) | length (mm) | Shape |
|---|---|---|---|---|
| M001361920 | Interlock - 18 Coil | 10 | 500 | 2D |
| M001361930 | Interlock - 18 Coil | 14 | 500 | 2D |
| M001361940 | Interlock - 18 Coil | 18 | 500 | 2D |
| M001361950 | Interlock - 18 Coil | 20 | 500 | 2D |
| M001361960 | Interlock - 18 Coil | 22 | 600 | 2D |
Diamond Configurations
| UPN | Description | Diameter (mm) | length (mm) | Shape |
|---|---|---|---|---|
| M001361740 | Interlock - 18 Coil | 2/3 | 230 | Diamond |
| M001361750 | Interlock - 18 Coil | 2/4 | 410 | Diamond |
| M001361760 | Interlock - 18 Coil | 2/5 | 580 | Diamond |
| M001361770 | Interlock - 18 Coil | 2/6 | 80 | Diamond |
* Bench testing performed by an independent laboratory intended to evaluate time required to occlude 75% of blood flow. Time to reach 75% cessation of blood flow distal to coil placement, measured at 1-minute time increments. Note: 75% cessation endpoint used since can’t measure flow in a hollow tube – 25% flow was minimum amount of flow able to be measured in model
Data one file at BSC. n=7 in Concerto group. N=9 in Ruby group. Bench test results may not necessarily be indicative of clinical performance.
Indications, contraindications, warnings and instructions for use can be found in the product labeling supplied with each device. Information for the use only in countries with applicable health authority product registrations Not for use or distribution in France