NC Emerge™
PTCA Dilatation Catheter
The Future Built on a Legacy
For decades, we have worked together to define the future. By bringing technology and performance together, we continue our commitment to evolving balloon catheter technology.
Key Resources
Explore
Product Details
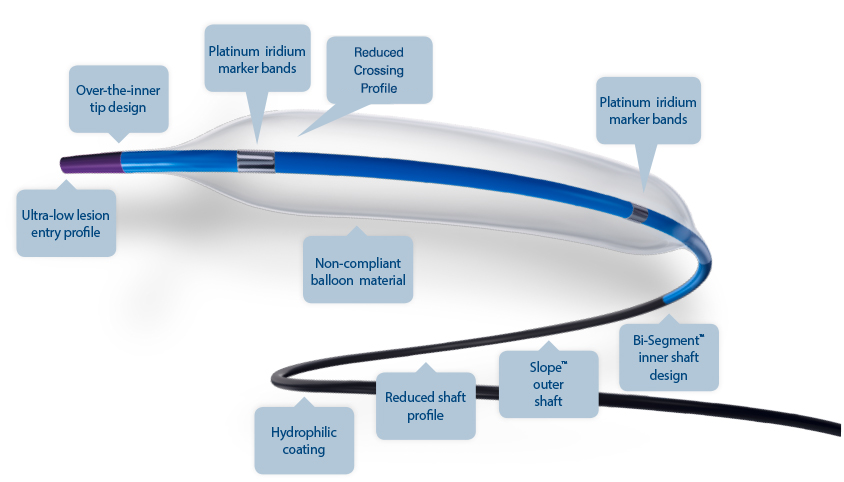
Addressing Balloon Growth and Deliverability Needs
Next Generation Non-Compliant Balloon
- Most non-compliant balloon of the devices tested1
Enhanced Deliverability
- Pre-dilatation profiles in a post-dilation balloon
- Ultra-low 0.017" (0.43mm) lesion entry profile
- Excellent tip flexibility enabling you to access your most complex lesions2
Excellent Simultaneous Use and Recross Performance
- Outstanding performance in simultaneous use compatibility and recrossability2
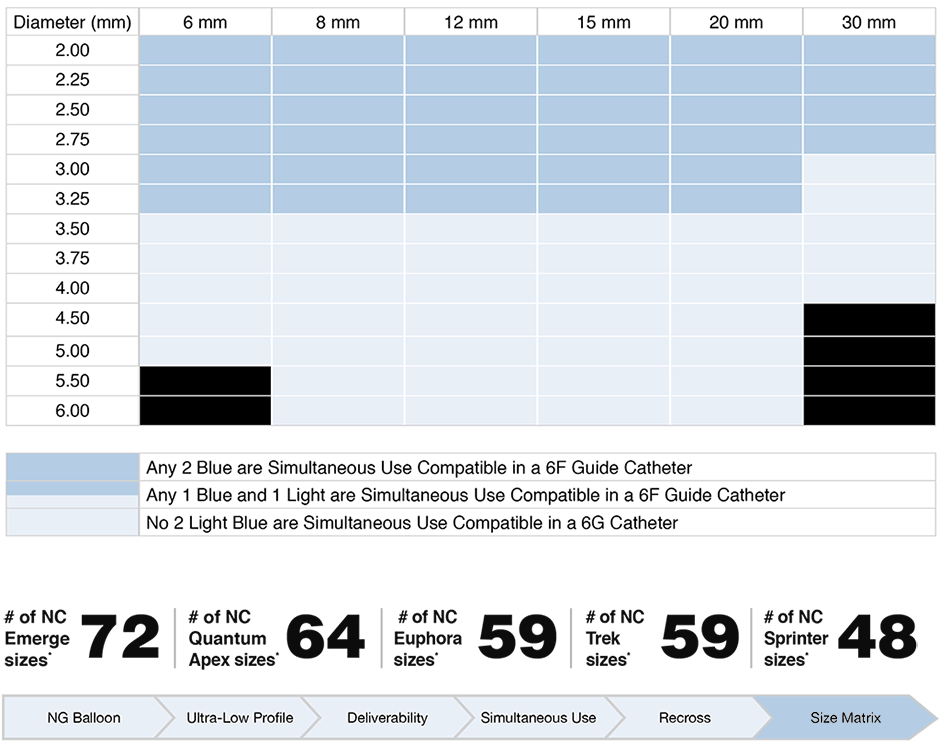
Additional Sizes - 5.5mm and 6.0mm
- Broadest size matrix on the market of the devices tested
NC Emerge is Boston Scientific's Most Advanced PTCA Catheter
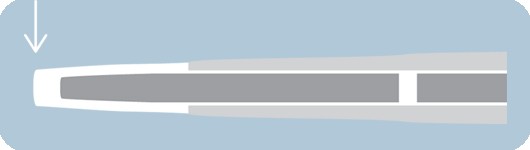
Ultra-low lesion entry point
- 0.017" lesion entry profile
- Improves overall flexibility and performance in tortuous anatomy
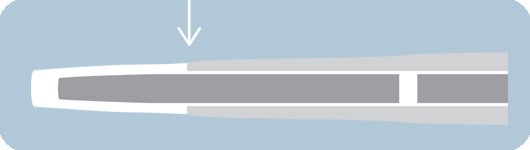
Over-the-inner tip design
- Outer tip material rides over the inner shaft
- Designed to improve overall flexibility and tip performance
- Short tip designed to lessen tip catch occurance and offer greater control
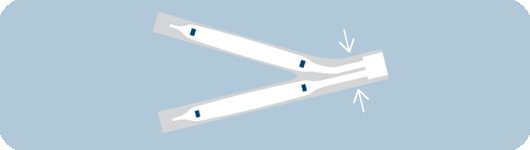
Reduced shaft profile
- Designed for exceptional simultaneous use performance
- Allows for use of two MonorailTM catheters in a 6 F
guide catheter and two Over-the-Wire catheters in an 8 F guide catheter3
Hydrophilic coating
- Reduced frictional force on the catheter shaft
Reduced crossing profile4
- 0.031" (0.787 mm) crossing profile
Bi-Segment™ inner shaft design
- Designed for maximum deliverability
- Both stiff and flexible segments to enhance pushability and trackability
Slope ™ outer shaft
- One piece outer shaft provides a seamless transition
- Designed to optimize pushability
- Slope outer shaft on OTW devices

Non-compliant balloon material
- Designed for less balloon growth and increased rated
burst pressure - Unique blend of balloon materials provides excellent
re-wrap
Platinum iridium marker bands
- Provides optimal radiopacity and excellent visibility
Product Size Matrix
Ordering Information
| Monorail | Balloon Length | |||||
| Balloon Diameter (mm) | 6 mm | 8 mm | 12 mm | 15 mm | 20 mm | 30 mm |
| 2 | H7493927606200 | H7493927608200 | H7493927612200 | H7493927615200 | H7493927620200 | H7493927630200 |
| 2.25 | H7493927606220 | H7493927608220 | H7493927612220 | H7493927615220 | H7493927620220 | H7493927630220 |
| 2.5 | H7493927606250 | H7493927608250 | H7493927612250 | H7493927615250 | H7493927620250 | H7493927630250 |
| 2.75 | H7493927606270 | H7493927608270 | H7493927612270 | H7493927615270 | H7493927620270 | H7493927630270 |
| 3 | H7493927606300 | H7493927608300 | H7493927612300 | H7493927615300 | H7493927620300 | H7493927630300 |
| 3.25 | H7493927606320 | H7493927608320 | H7493927612320 | H7493927615320 | H7493927620320 | H7493927630320 |
| 3.5 | H7493927606350 | H7493927608350 | H7493927612350 | H7493927615350 | H7493927620350 | H7493927630350 |
| 3.75 | H7493927606370 | H7493927608370 | H7493927612370 | H7493927615370 | H7493927620370 | H7493927630370 |
| 4 | H7493927606400 | H7493927608400 | H7493927612400 | H7493927615400 | H7493927620400 | H7493927630400 |
| 4.5 | H7493927606450 | H7493927608450 | H7493927612450 | H7493927615450 | H7493927620450 | |
| 5 | H7493927606500 | H7493927608500 | H7493927612500 | H7493927615500 | H7493927620500 | |
| 5.5 | H7493927608550 | H7493927612550 | H7493927615550 | H7493927620550 | ||
| 6 | H7493927608600 | H7493927612600 | H7493927615600 | H7493927620600 | ||
1.Growth as measured from compliance charts on directions for use for 3.0mm x 15mm balloon
2.Testing completed by Boston Scientific Corp. N=15 Data on file. Bench test result may not necessarily be indicative of clinical performance.
3.6 F guide catheter with a minimum 0.070" ID, 8 F guide catheter with a minimum 0.088" ID
4.Crossing profile is defined as the maximum diameter found between the proximal end of the balloon and the distal tip of the catheter. Definition excerpted from FDA Guidance document titeled, Class II Special Controls Guidance Document for Certain Percutaneous Transluminal Coronary Angioplasty (PTCA) Catheters.


















