Discover the potential of intravascular insight
Enhance endovascular procedures by providing real-time, high-resolution vessel assessment, leading to better clinical outcomes.
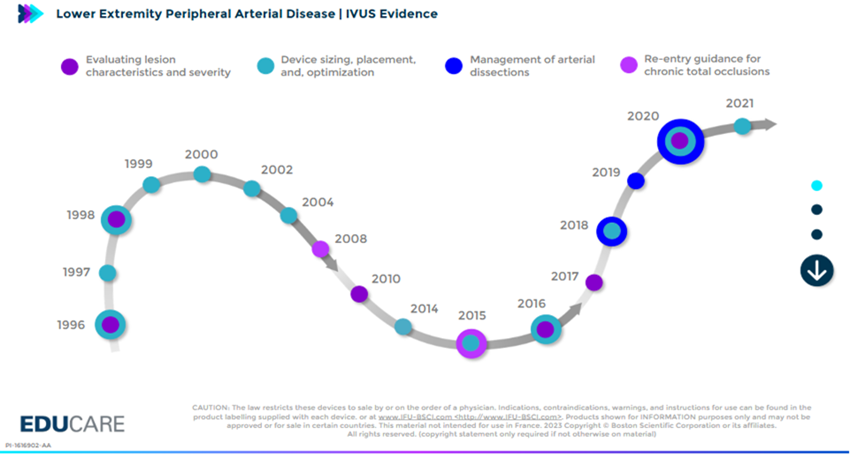
Studies show that compared to angiography alone, Intravascular Ultrasound (IVUS) improves primary patency, reduces binary restenosis, and minimizes the need for bailout stenting by enabling accurate lesion morphology analysis and vessel sizing for optimal drug delivery.
SEE more with IVUS
Improved vessel sizing and lesion assessment
led to a change in treatment strategy in
79%
of cases.¹
SEE more to improve DCB outcomes
An international panel of 40 vascular experts established consensus to encourage, standardize and optimize the use of IVUS². This approach is increasingly supported by leading voices in the field, who emphasize that IVUS and angiography are complementary procedures: together they provide a more complete picture for informed peripheral endovascular decisions³.
Clinical Evidence highlights the value of IVUS:
- Reduces stenting rates.⁴
- Lowers restenosis, reintervention, and limb events in femoropopliteal disease.5-7
- Detection of 4 - 5 times more dissections than conventional angiography. ⁸
SEE more with IVUS makes a difference in long term outcomes and benefits are greater in more complex lesions. ⁹
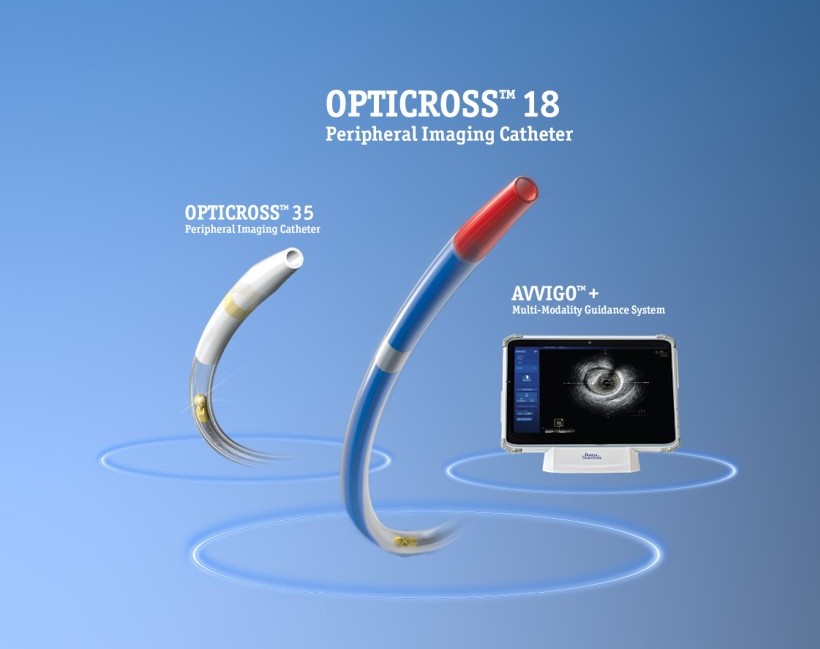
Advanced devices for intravascular clarity
Our SEE more portfolio brings together cutting-edge imaging technologies designed to enhance precision and clarity throughout the procedure.
Informed decisions with OPTICROSS™ 18
Unmatched clarity. Confident decisions. The best-in-class deliverability – enables crossing challenging lesions while providing exceptional image quality.
AVVIGO™+
Multi-Modality Guidance System
Access the product page
OPTICROSS™ 18
Peripheral imaging catheter
Access the product page
OPTICROSS™ 35
Peripheral imaging catheter
Access the product page
Your Modern Endovascular Interventions learning journey
Explore our Endovascular Interventions Online Learning platform for free online education and find in-person training events to SEE more and keep vessels open for longer.
References:
1. Allan et al., The Impact of Intravascular Ultrasound on Femoropopliteal Artery Endovascular Interventions, JACC Cardiovascular Intervention, 2022
2. IVUS in Peripheral Interventions: Clinical Consensus and Recommendations, as presented by Eric Secemsky (Boston, MA, USA) at Vascular Interventional Advances (VIVA), 5-7 October 2021.
3. Secemsky et al., Intravascular Ultrasound Use in Peripheral Arterial and Deep Venous Interventions: Multidisciplinary Expert Opinion From SCAI/AVF/AVLS/SIR/SVM/SVS. J Vasc Interv Radiol, 2024
4. PENDING CONFIRMATION
5. Allan RB et al. The Impact of Intravascular Ultrasound on Femoropopliteal Artery Endovascular Interventions: A Randomized Controlled Trial. JACC Cardiovasc Interv. 2022 Mar 14;15(5):536-546. doi: 10.1016/j.jcin.2022.01.001. PMID: 35272779.
6. Jang et al., Meta-analysis of Intravascular Ultrasound-Guided Versus Angiography Guided Endovascular Treatment in Lower Extremity Artery Disease, The American Journal of Cardiology, 2025
7. Lee et al., Intravascular Ultrasound-Guided vs Angiography-Guided Drug-Coated Balloon Angioplasty in Patients With Complex Femoropopliteal Artery Disease, JACC: Cardiovascular Interventions, 2025.
8. Shammas NW, Torey JT, Shammas WJ et al. Intravascular ultrasound assessment and correlation with angiographic findings demonstrating femoropopliteal arterial dissections post atherectomy: Results from the iDissection Study. J Invasive Cardiol. 2018;30(7):240-244.
9. Ko Young-Guk et al; IVUS-DCB investigators. Intravascular ultrasound-guided drug-coated balloon angioplasty for femoropopliteal artery disease: a clinical trial. Eur Heart J. 2024 Aug 16;45(31):2839-2847. doi: 10.1093/eurheartj/ehae372. PMID: 38966936.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.