The TARGET Study
A global real-world retrospective study that confirms TheraSphere for HCC as safe and effective, demonstrating predictable clinical outcomes across a broad patient population in 8 countries.
Lam, M., Garin, E., Maccauro, M. et al. A global evaluation of advanced dosimetry in transarterial radioembolisation of hepatocellular carcinoma with Yttrium-90: the TARGET study. Eur J Nucl Med Mol Imaging (2022). https://doi.org/10.1007/s00259-022-05774-0
Study Objective
Establish the relationships between:
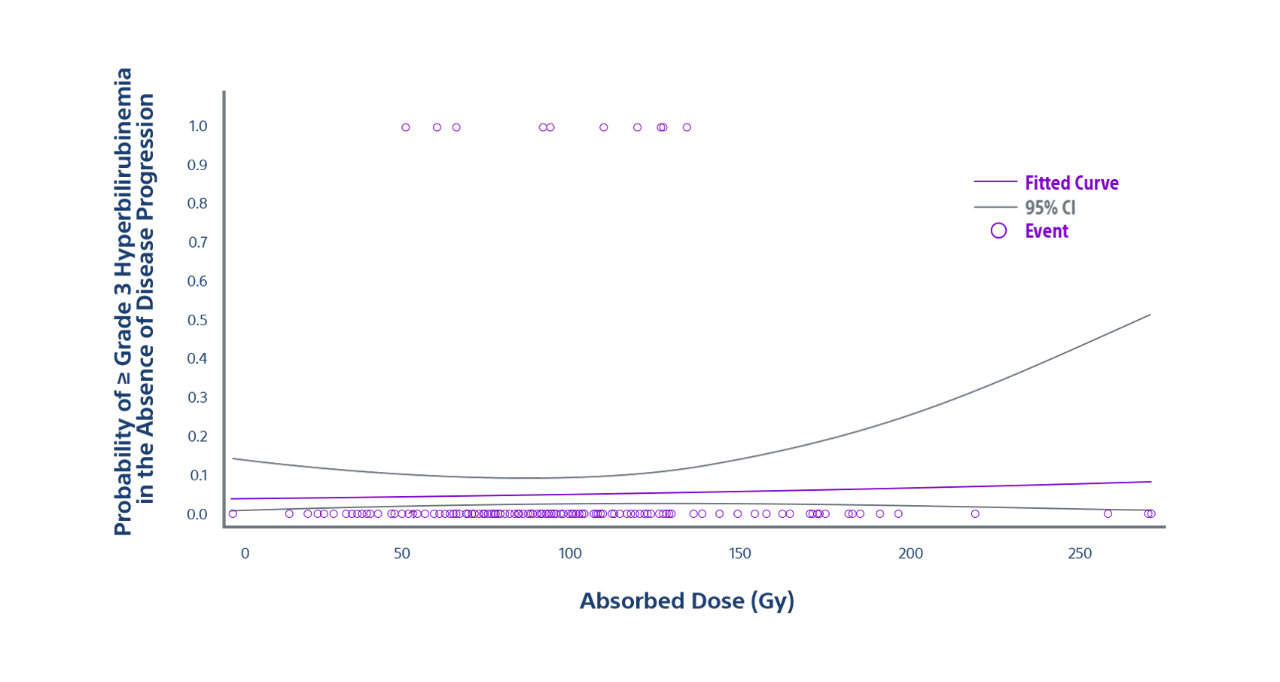
- Normal tissue adsorbed dose (NTAD) and occurrence of grade 3 or higher hyperbilirubinemia
- Tumour absorbed dose (TAD) and Objective Response Rate (ORR)
- TAD and Overall Survival (OS)
Study Design
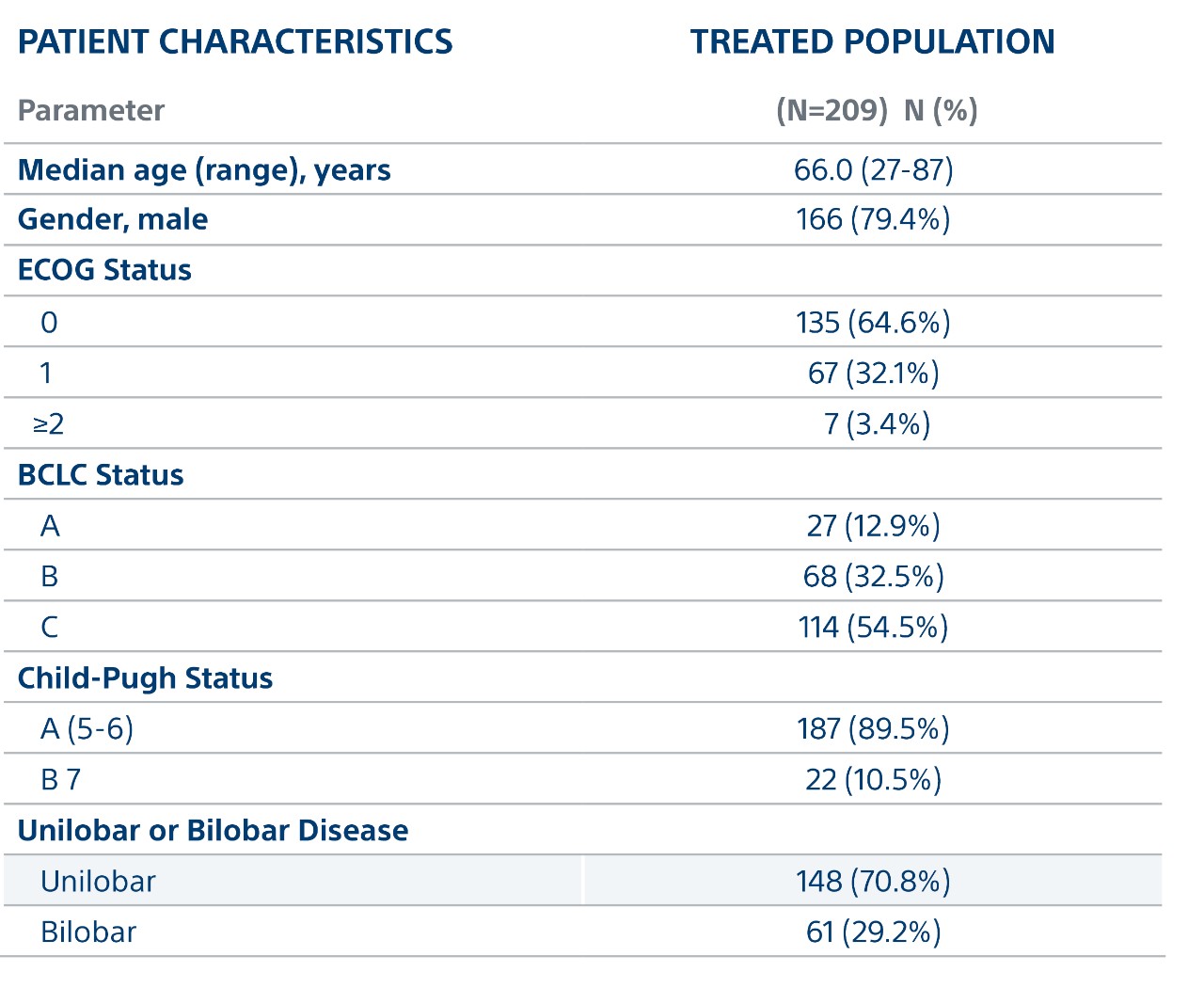
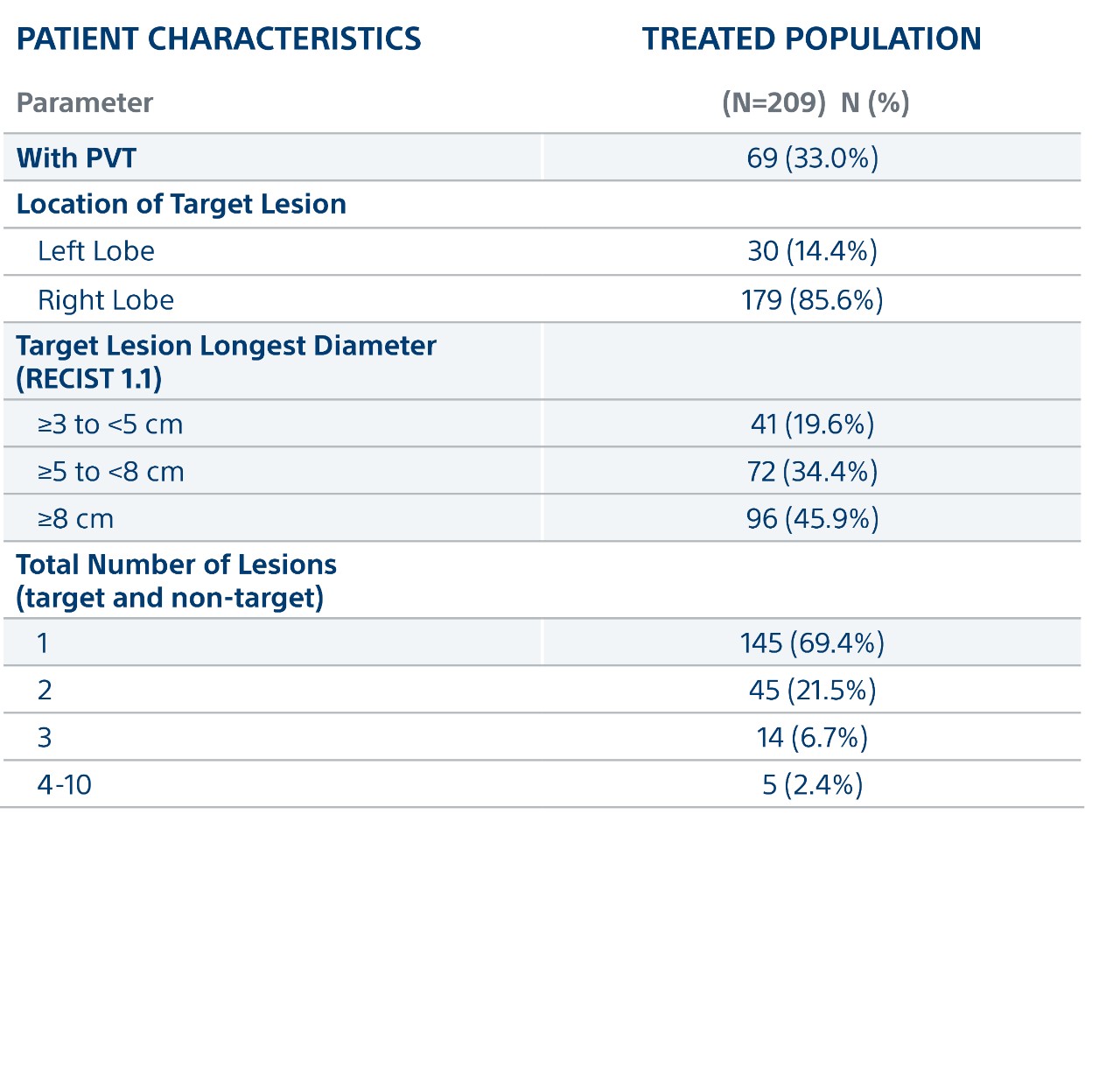
Key Patient Characteristics
- Mainly Intermediate and Advanced HCC: 33% BCLC B and 55% BCLC C
- 7cm median target lesion
- 33% PVT
Dosimetry Approach
- Investigator review of patient chart and dosimetry calculation
- Retrospective dosimetry evaluation with multi-compartment approach using Simplicit90Y™ personalised dosimetry software to determine TAD and NTAD
Results
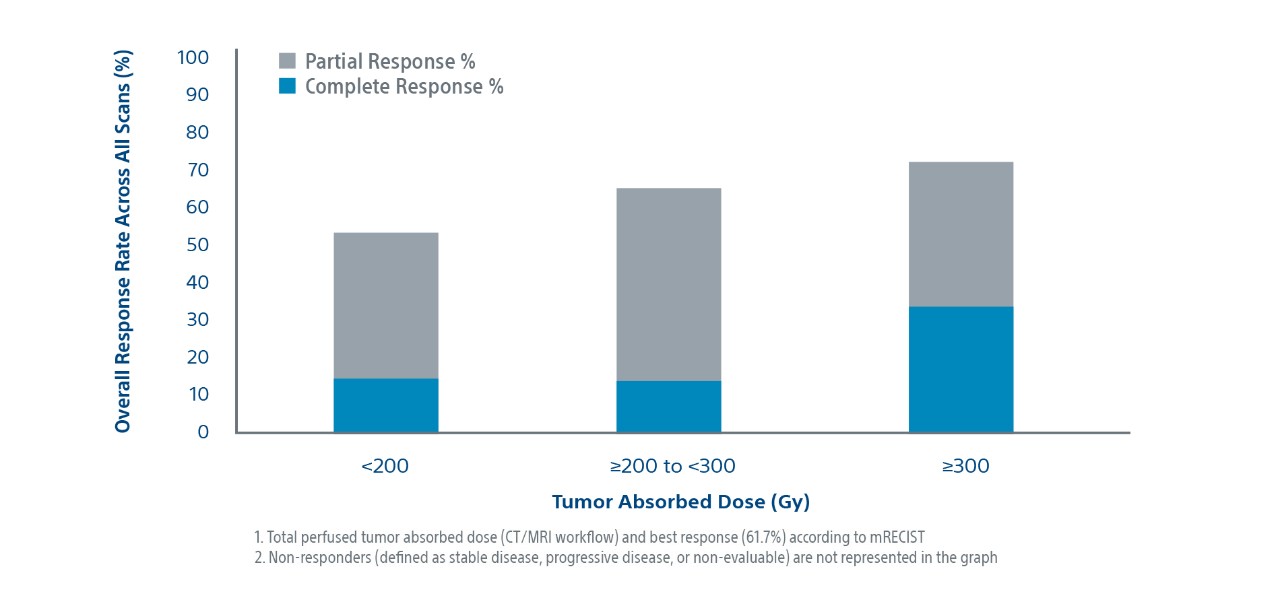
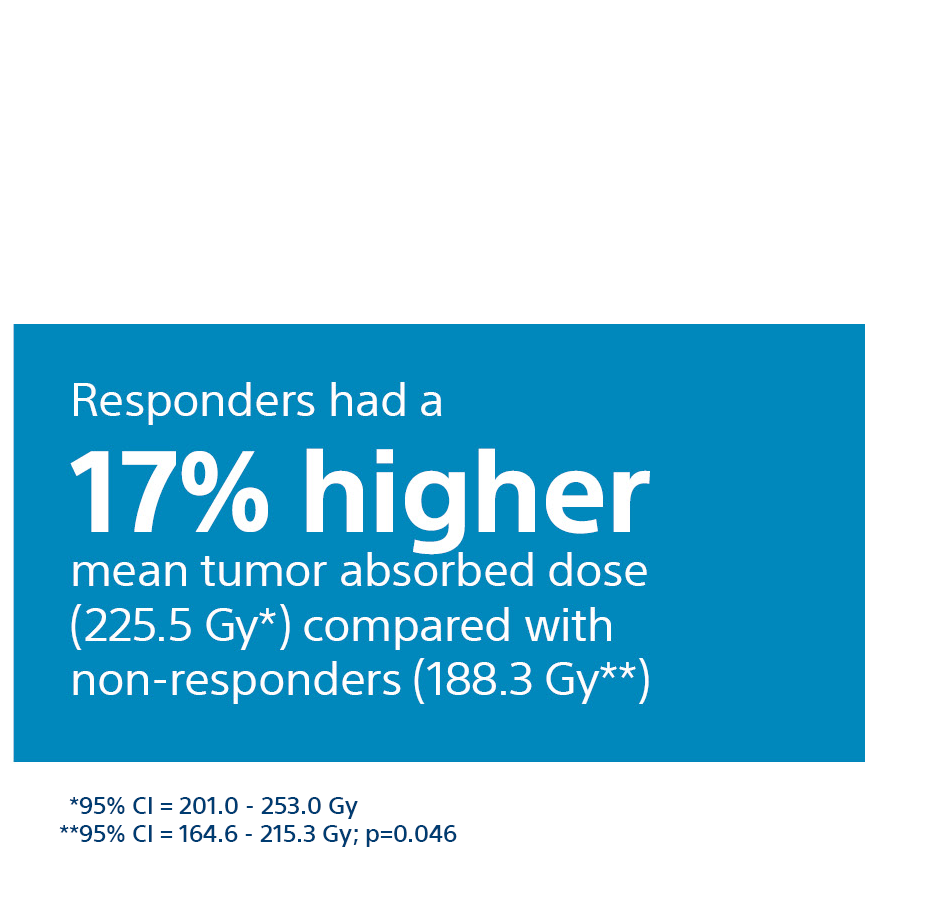
Tumour Absorbed Dose Was Predictive of Response1,2
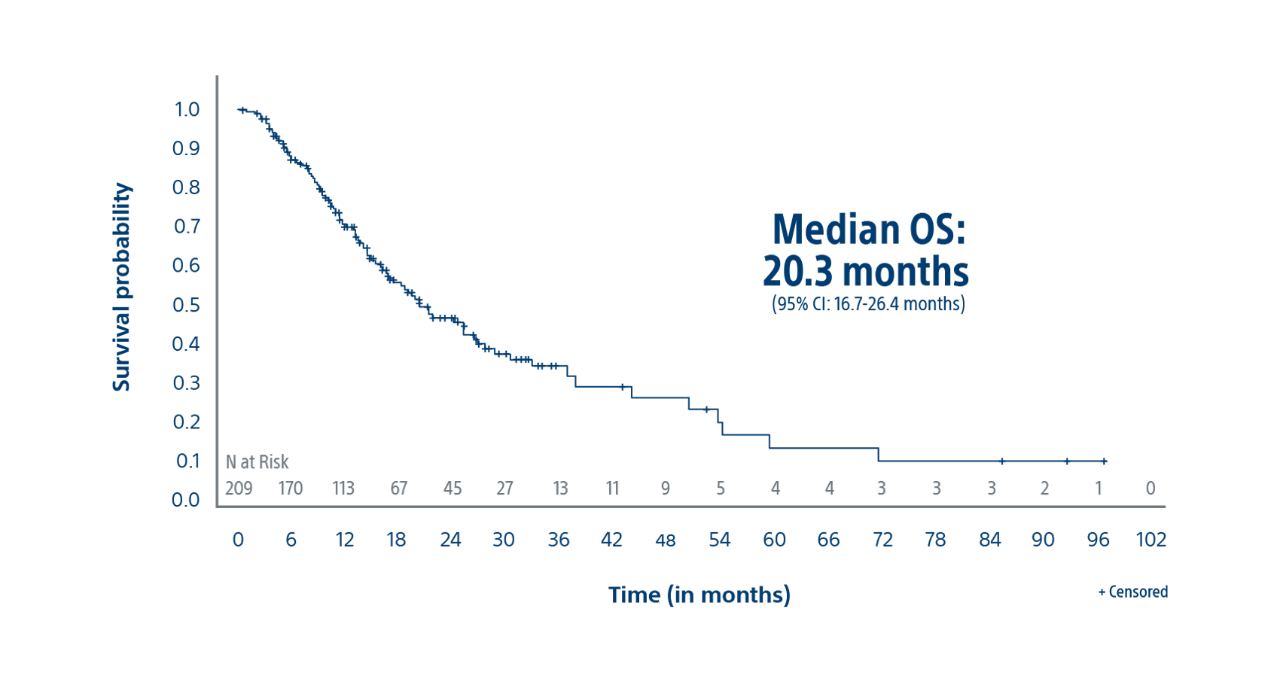
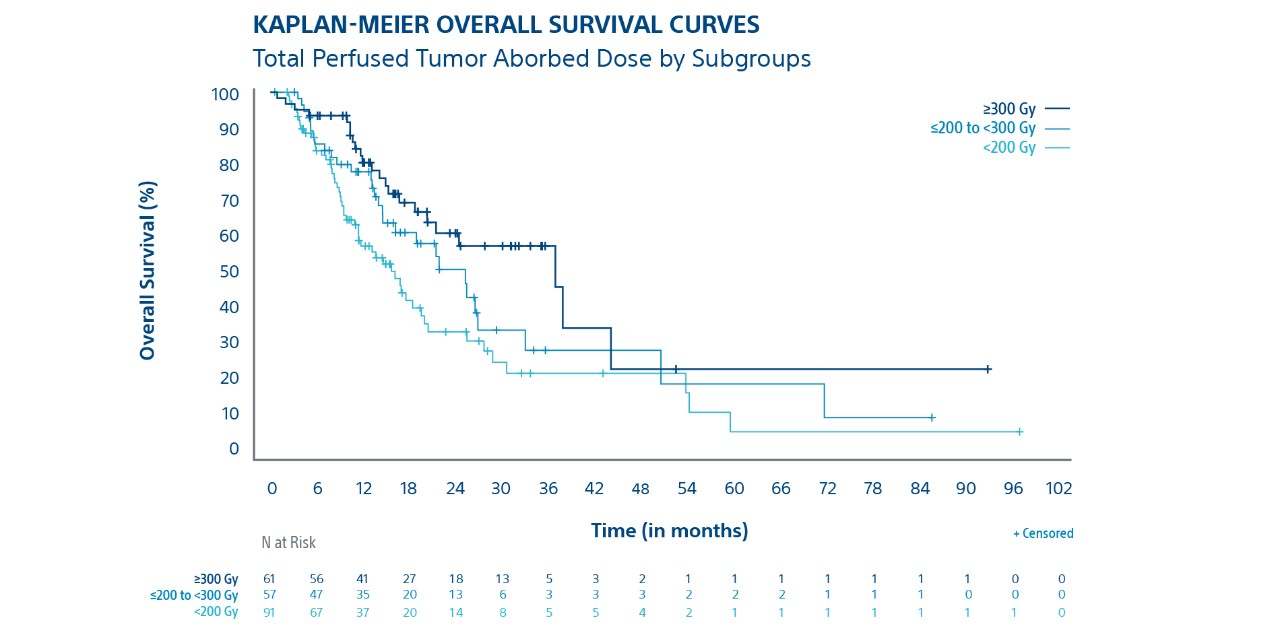
Tumour Absorbed Dose Was Predictive of Overall Survival
Low Rate of ≥ Grade 3 Hyperbilirubinemia Confirms Safety of TARGET Study

Study Takeaways
|
|
|

Perform SIRT with TheraSphere™

SIMPLICIT90Y™
Embrace Y90
SIRT Personalised Dosimetry
SIRT Personalised Dosimetry

![]()
Virtual Events
View our latest virtual symposia and webinars on interventional oncology topics.

![]()
Stay up to date
Receive emails about the latest interventional oncology news, innovations and events.