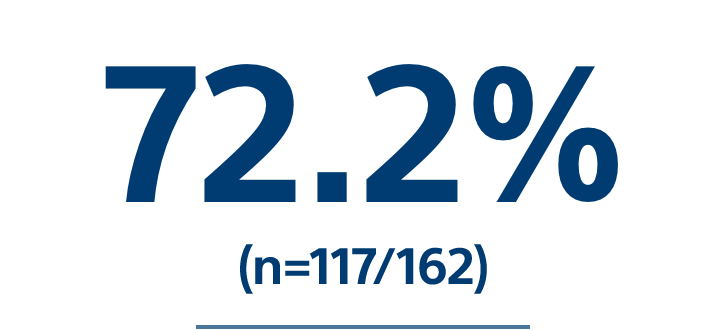Interventional Oncology LEGACY Study

The LEGACY study is a robust multi-centre study confirming TheraSphereTM as a neoadjuvant or
standalone therapy in treating HCC.*
standalone therapy in treating HCC.*
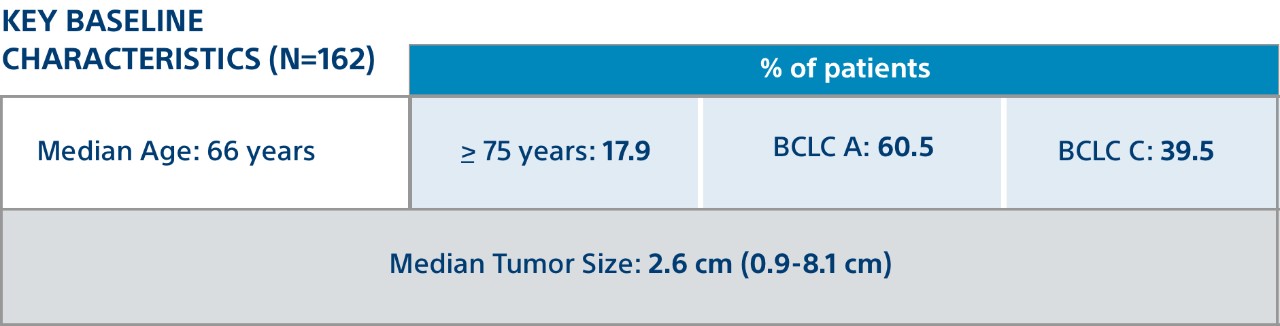
Included 162 consecutive treated patients with HCC |
Presented by Riad Salem, MD at CIRCE 2020.

Exceeding the Standards of Care
"We studied Y-90 in HCC patients at all stages of the disease and we found that it was either equal to or better than the current standards of care but providing a much more gentle treatment for patients. And that was enough to seal the deal."
Prof. Riad Salem, MD an Interventional Radiologist at Northwestern Memorial Hospital

Study Objective
To assess local tumor control and duration of response following treatment with Y-90 glass microspheres in patients with unrespectable solitary HCC lesions.
Key Study Results
|
|
|
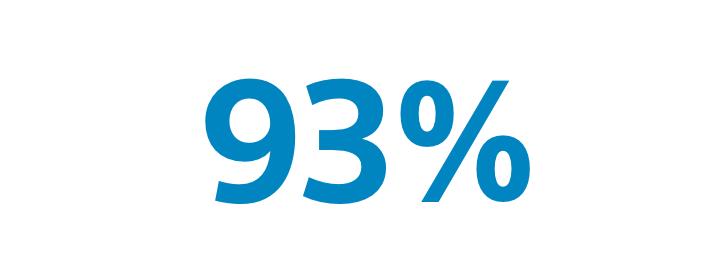
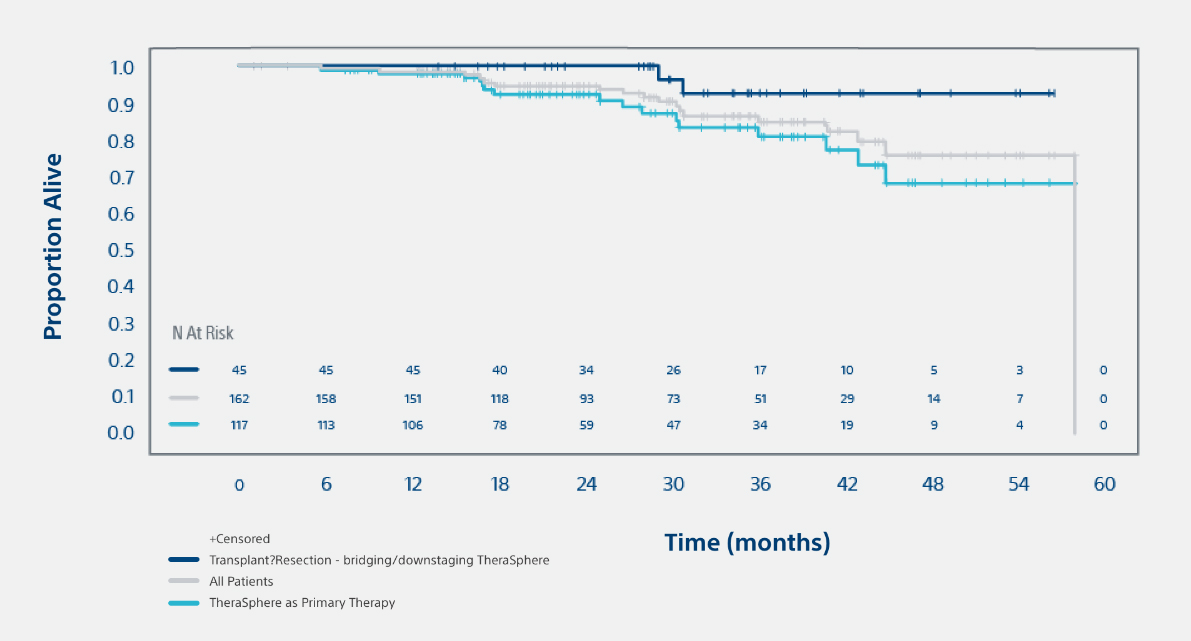
Overall Survival(Treated population)93% overall survival rate in patients with transplant or resection following TheraSphereTM at 3 years. |
 |

Tumor Response(Best Response in evaluable population, localized mRECIST) |
 |
Study Design:
Multi-center, single-arm, retrospective study conducted at 3 U.S. sites.** Consecutive patients meeting the eligibility criteria were treated with TheraSphereTM Y-90 Glass Microspheres at each site between January 2014 and December 2017.
First and Only:
- Used highly clinically relevant criteria for localized tumor control (mRECIST)
- Reported a median dose to perfused liver volume of 410 Gy
- Demonstrated 100% of patients achieved CR or PR (localized mRECIST)
Key Eligibility Criteria:
Unresectable solitary lesions (≤ 8 cm); Selective, lobar, or mixed administration of Y-90 glass microspheres (TheraSphereTM); Treatment purpose (neoadjuvant to transplantation or resection or stand-alone treatment); Child-Pugh score A; BCLC A or BCLC C (ECOG 1); No prior liver transplantation, resection, locoregional treatment or systemic therapy; No portal vein thrombosis or extrahepatic disease.
Primary Study Endpoints Were MetDetermined by Blinded Independent Central Review (BICR) |
||||||
|
||||||
| Safety: Majority of adverse events were mild and resolved without medical intervention. |

LEGACY STUDY CONCLUSION:
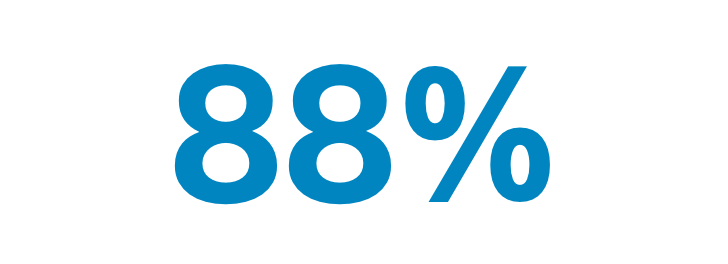
LEGACY is the first multi-centre study to report a high median perfused volume absorbed dose of 410 Gy with TheraSphereTM, which resulted in an 88% best response, excellent and durable tumor control and high overall survival rate in patients with early and advanced HCC.
*Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, Fowers K, Lewandowski R, Padia SA. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable Hepatocellular Carcinoma: The LEGACY Study. Hepatology. 2021 Mar 19. doi: 10.1002/hep.31819.
**University of Washington, Seattle, WA; Northwestern University, Chicago, IL; Mount Sinai Health System, New York, NY
1. Complete Response (CR) and Partial Response (PR) within the treatment area according to localized mRECIST
2. Duration of Response (DoR) According to localized mRECIST
3. Median follow-up was 29.9 months [95% CI: 24.7, 34.6]

Perform SIRT with TheraSphere™

SIMPLICIT90Y™
Embrace Y90 SIRT
Personalised Dosimetry
Personalised Dosimetry

![]()
Virtual Events

![]()