Medical Specialties > Interventional Radiology > Interventional Oncology > Latest evidence > EPOCH Trial > Data Sheet Download
Interventional Oconlogy EPOCH Trial data sheet
An international, multi-centre, open-label, phase III, randomised controlled trial to study the impact of TheraSphere™ Y-90 Glass Microspheres in combination with second-line systemic chemotherapy for colorectal liver metastases (CLM)
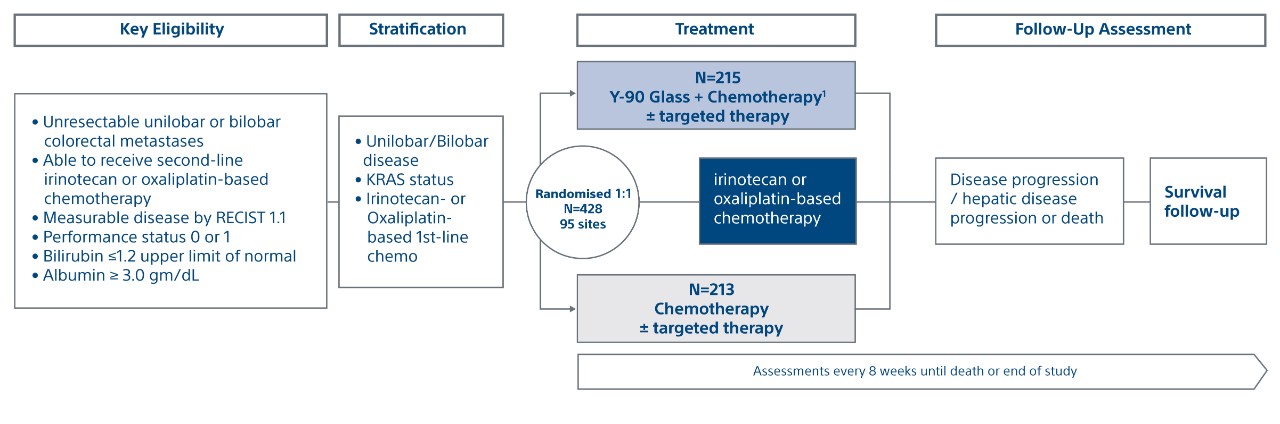
Phase III, open label, multicentre (95 sites), prospective, 1:1 randomised trial.
Primary endpoints:
- PFS according to RECIST 1.1 from time of randomization to disease progression or death
- hPFS according to RECIST 1.1, from time of randomization to thedate of disease progression in the liver or death















