EPOCH Trial
EPOCH is a level 1, phase III randomised controlled trial using transarterial radiation therapy for mCRC liver metastases that demonstrated statistically significant improvements in both Progression-Free Survival (PFS) and Hepatic Progression-Free Survival (hPFS) in patients who progressed on first-line chemotherapy.
Trial Objective & Design
Trial Objective
To evaluate the safety and efficacy of TheraSphere Y-90 Glass Microspheres combined with second-line therapy (oxaliplatin- or irinotecan-based chemotherapy) in patients with mCRC of the liver.
Trial Design
An open-label, prospective, multicenter, phase III trial of 428 patients randomised 1:1 to treatment arm (TheraSphere + second-line chemotherapy) vs. control arm (second-line chemotherapy alone) across 95 centers in 12 countries, including North America, Europe and Asia.
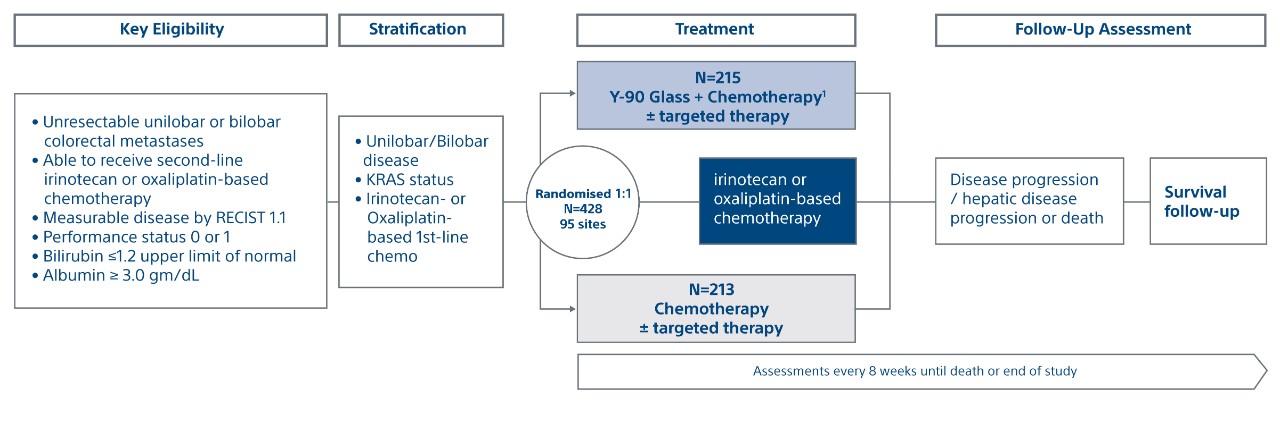
Unilobar or Bilobar Disease, KRAS Status (Mutant or Wild type, 1st Line Chemo (Irinotecan or oxaliplatin based)
Primary Endpoints
Progression-free survival (PFS) and hepatic PFS (hPFS)
- Time from randomisation to progression or death by RECIST 1.1 or death
- Blinded independent central review (BICR)
Both primary endpoints successfully met
TheraSphere Y-90 Glass Microspheres used in combination with chemotherapy as a second-line treatment demonstrated statistically significant improvements in both PFS and hPFS in patients who progressed on first-line chemotherapy.
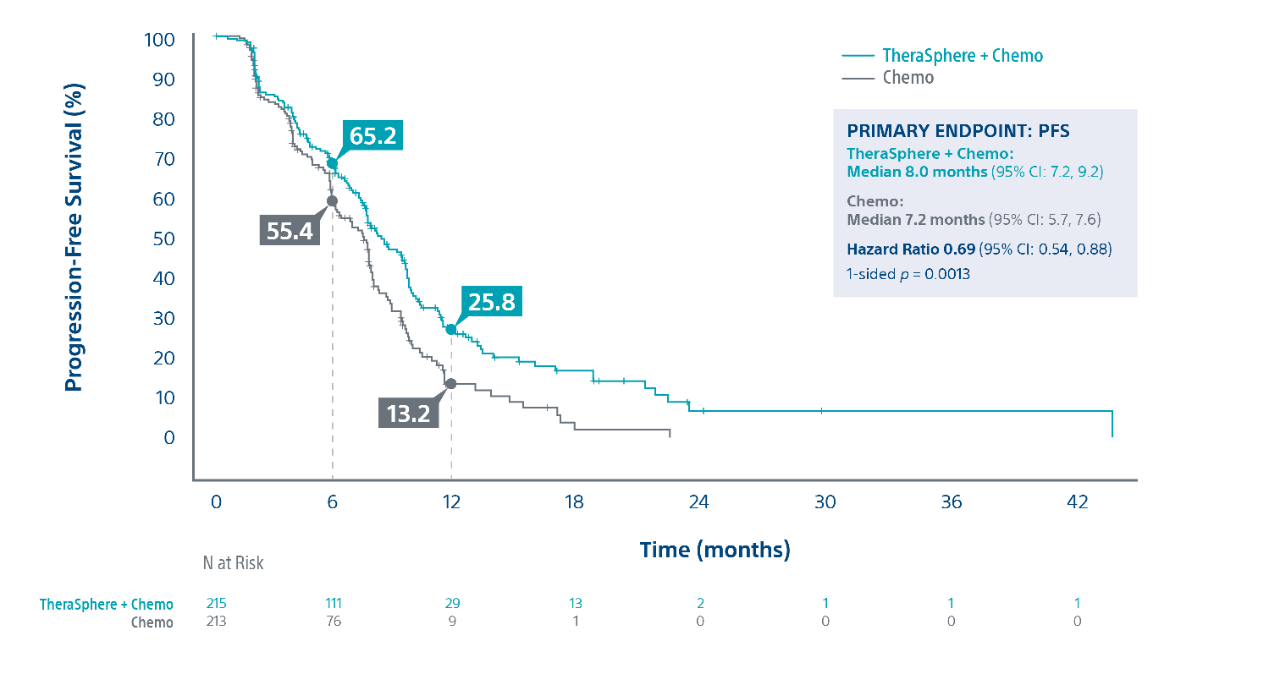
Progression Free Survival2
 |
Patients receiving TheraSphere with second-line chemo were 31% less likely to experience disease progression or death (due to any cause) vs. chemo alone. |
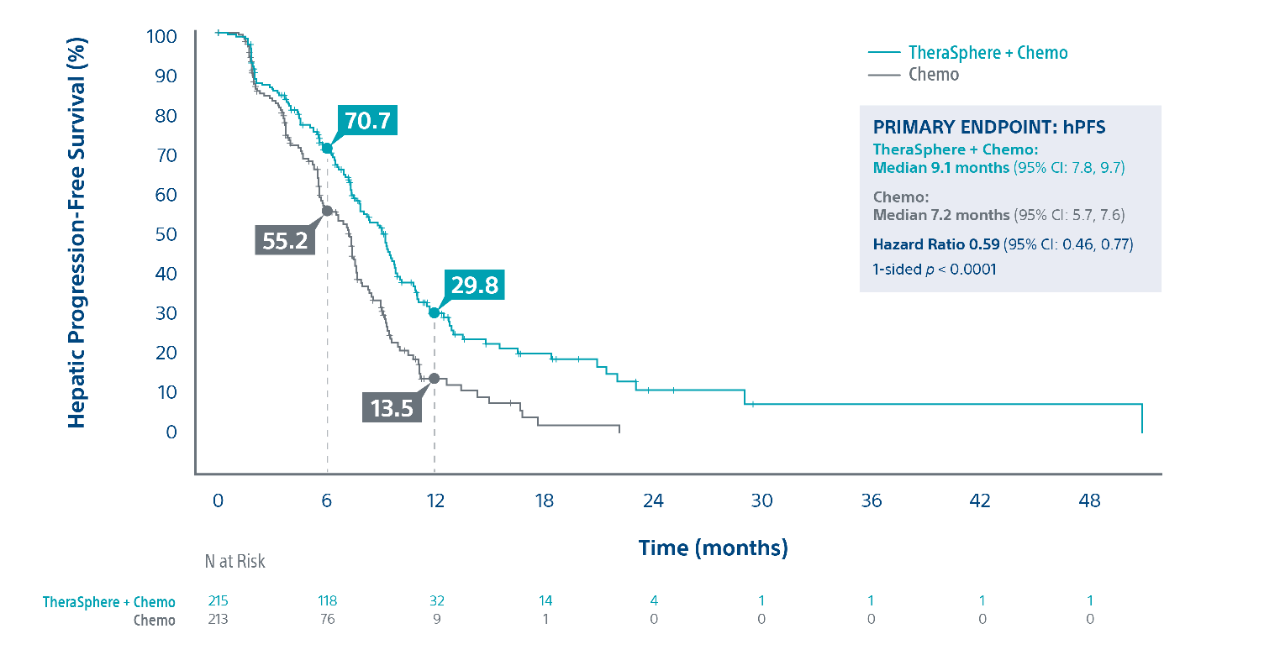
Hepatic Progression Free Survival3
 |
Patients receiving TheraSphere with second-line chemo were 41% less likely to experience hepatic disease progression or death (due to any cause) vs. chemo alone. |
Secondary Endpoints
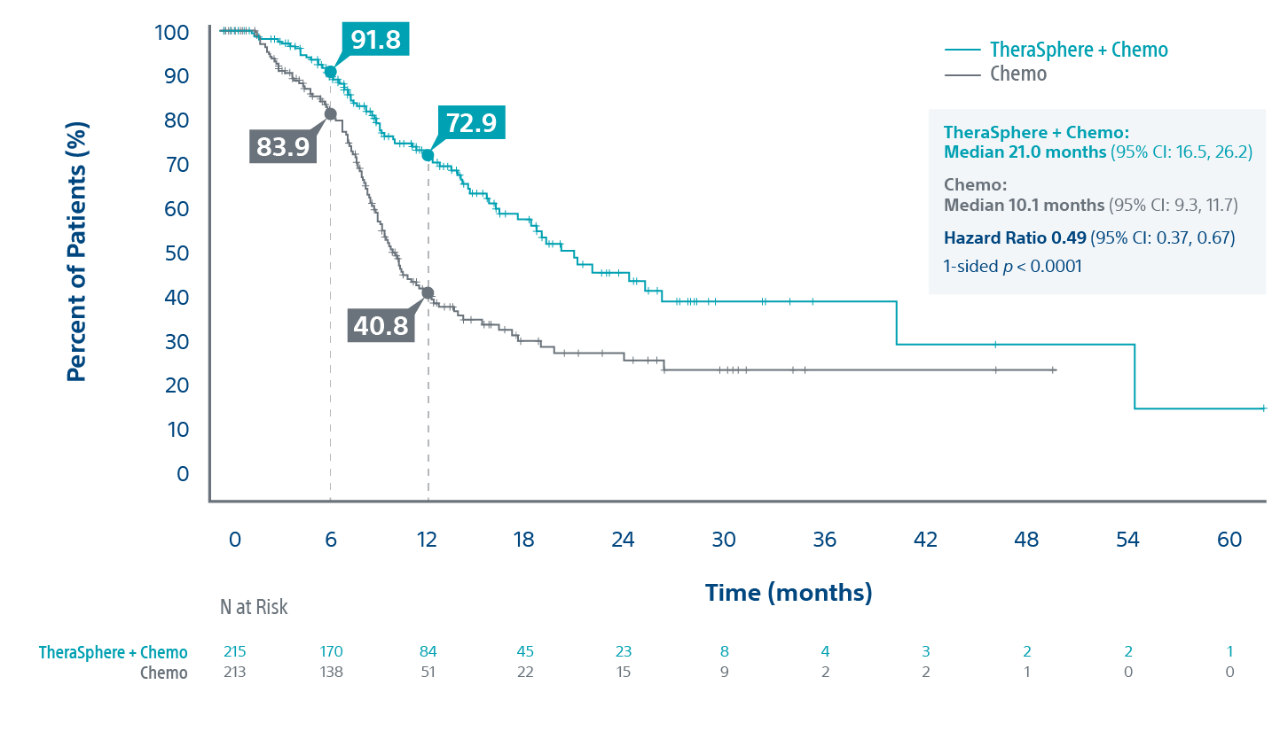
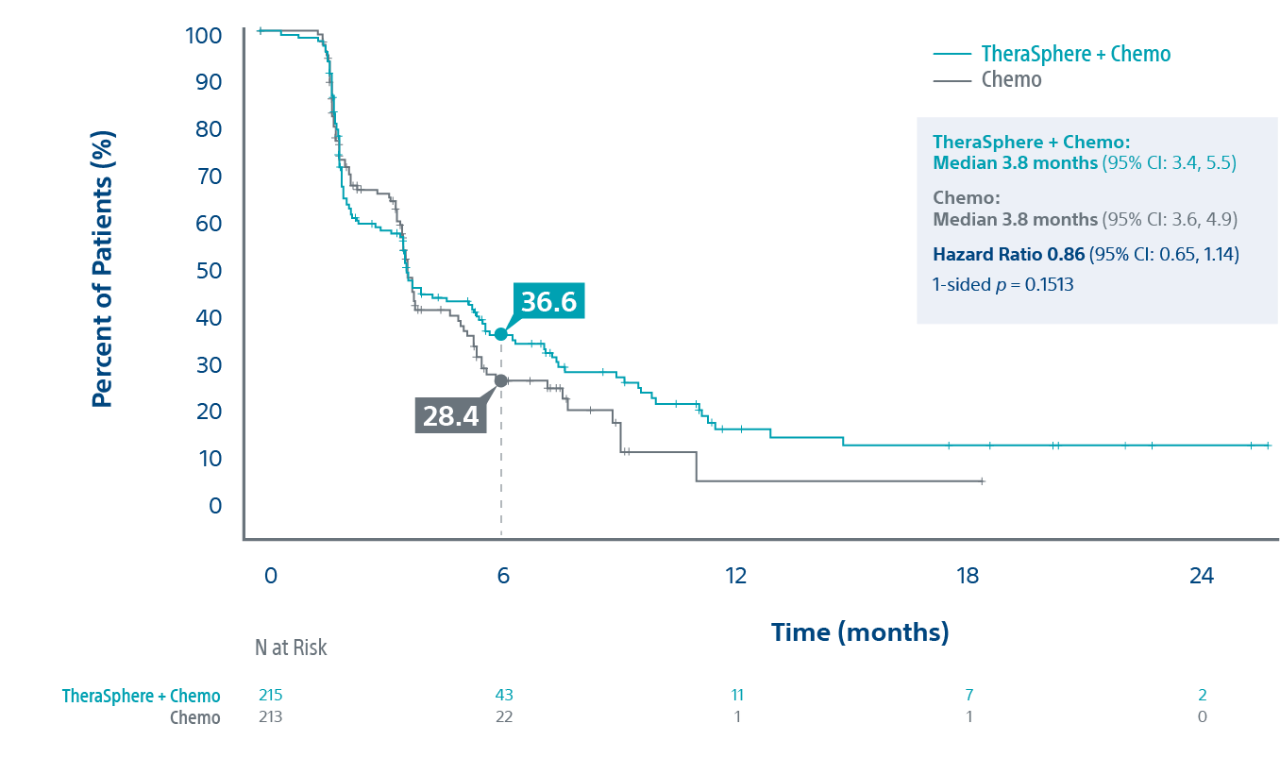
Median Overall Survival (in months):
- Intent-to-Treat (ITT): 14.0 vs. 14.4 for TS+Chemo (N=215) vs. Chemo alone (N=213) (1-sided p-value: 0.7229)
- Per-Protocol (PP): 15.2 vs. 14.3 for TS+Chemo (N=115) vs. Chemo alone (N=173) (1-sided p-value: 0.3841)*
* TS+Chemo (N=100) and Chemo alone (N=40) patients excluded from Per Protocol analysis due to major deviations
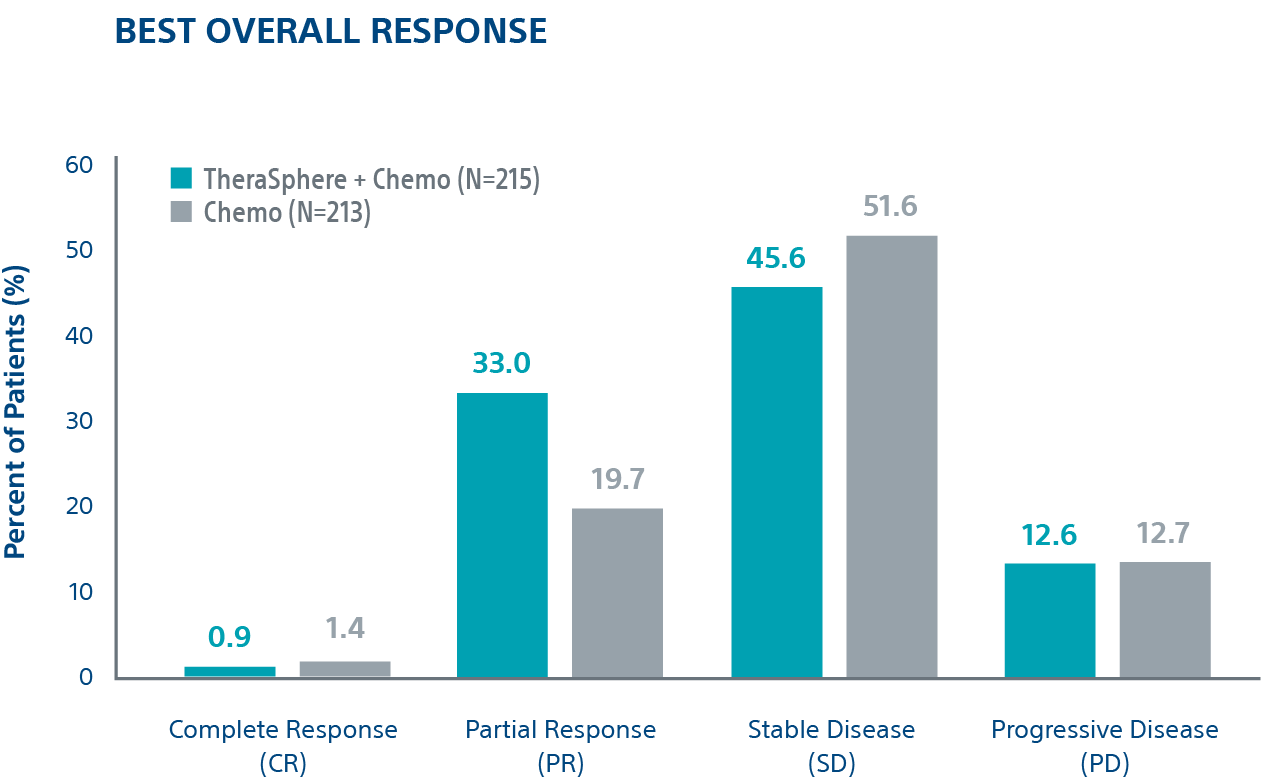
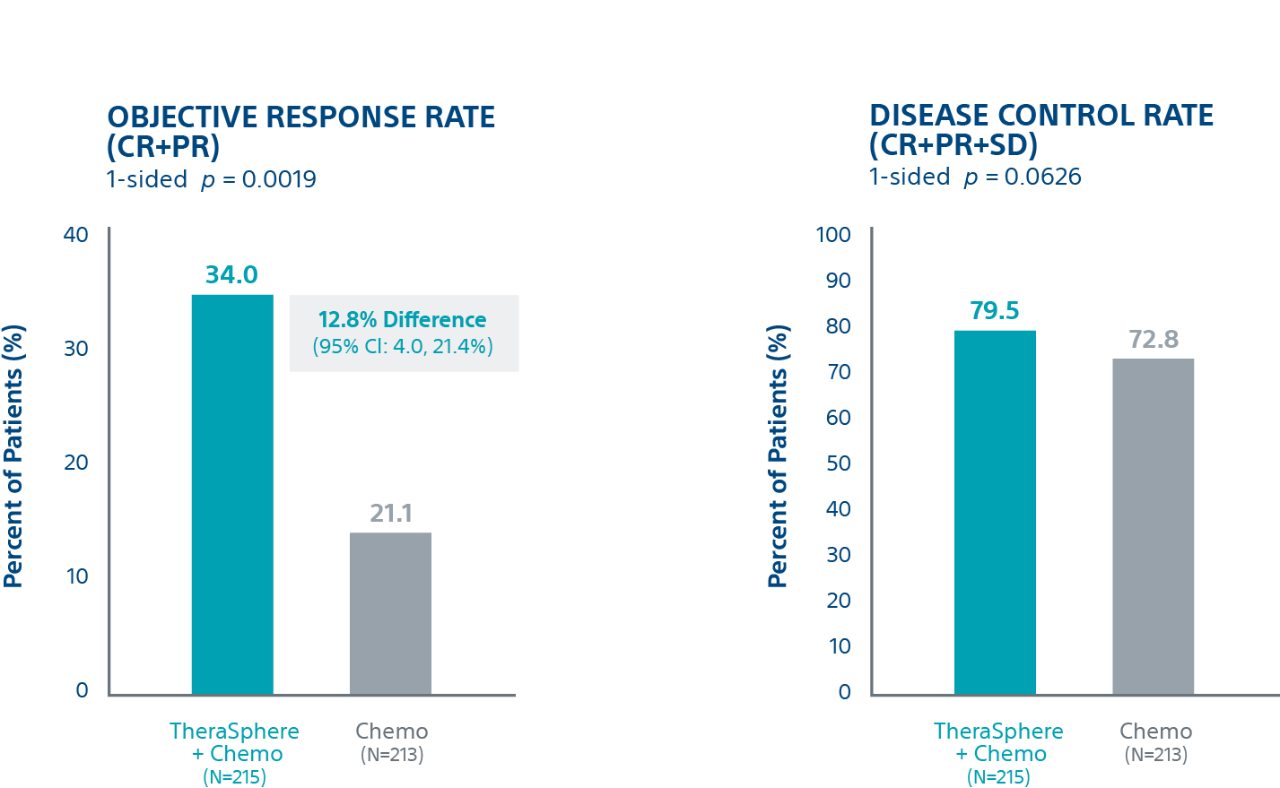
Tumour Response4
Patients receiving TheraSphere Y-90 with second-line chemotherapy showed an Objective Response Rate (ORR) of 34.0% vs. 21.1% for the control arm; a difference of 12.8%.
Additional Analyses
Time to Start of Subsequent Therapy & Quality of Life
The addition of TheraSphere Y-90 to second-line chemotherapy extended the time to start of subsequent therapy without compromising quality of life.
Time to Start of Subsequent Therapy5
Time to Deterioration of Quality of Life6
Key Patient & Disease Characteristics
| TheraSphere + Chemo (N = 215) | Chemo (N = 213) | |
| Median Age (y) | 63.0 | 60.0 |
| Male | 135 (62.8%) | 138 (64.8%) |
| Region North America Europe Asia | 63 (29.3%) 131 (60.9%) 21 (9.8%) | 56 (26.3%) 145 (68.1%) 12 (5.6%) |
| ECOG 0 | 119 (55.3%) | 133 (62.4%) |
| Albumin ≥ Site LLN | 182 (84.7%) | 177 (83.1%) |
| CEA ≥ 35ng/mL | 116 (54.0%) | 105 (49.3%) |
| KRAS Status Mutant Wild Type | 100 (46.5%) 115 (53.5%) | 101 (47.4%) 112 (52.6%) |
| Bilobar disease | 176 (81.9%) | 173 (81.2%) |
| Liver tumor burden at baseline by BICR < 10% ≥ 10% to < 25% ≥ 25% Missing | 124 (57.7%) 54 (25.1%) 29 (13.5%) 8 (3.7%) | 121 (56.8%) 47 (22.1%) 28 (13.1%) 17 (8.0%) |
| Maximum liver lesion size ≥ 4cm | 162 (75.3%) | 142 (66.7%) |
| Primary tumor in situ | 83 (38.6%) | 69 (32.4%) |
| Left side primary tumor location | 150 (69.8%) | 136 (63.8%) |
| Extrahepatic metastases at baseline | 113 (52.6%) | 95 (44.6%) |
Treatment Characteristics
| TheraSphere + Chemo (N = 215) | Chemo (N = 213) | |
| Received Assigned Therapy | 187 (87.0%) | 191 (89.7%) |
| 2nd Line Chemo Administered | 203 (94.4%) | 191 (89.7%) |
| Irinotecan-based / Mean Number of Cycles | 130 (60.5%) / 9.0 | 123 (57.7%) / 9.5 |
| Oxaliplatin-based / Mean Number of Cycles | 73 (34.0%) / 8.5 | 68 (31.9%) / 8.8 |
| Biological Agent | 88 (40.9%) | 93 (43.7%) |
| TheraSphere Y-90 Glass Microspheres Treatment | ||
|---|---|---|
| Median time to Y-90, days (range) | 25 (12, 90) | NA |
The addition of TheraSphere Y-90 to second-line chemotherapy did not increase chemo-related adverse events and no new safety signals were identified.1