Dosisphere-01 Trial Summary
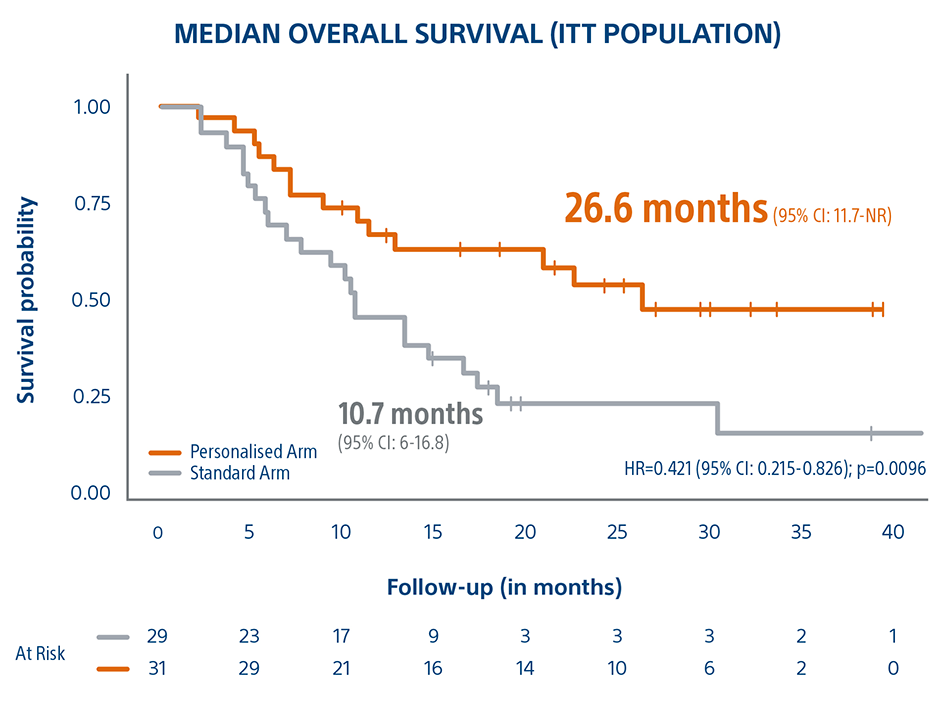
This Level 1 clinical trial demonstrated that personalized TheraSphere dosimetry, using multicompartamental dose administration, achieved a 26.6 month median OS for large tumor HCC patients and a 16-month survival improvement compared to the control arm on standard dosimetry.
Garin E, Tselikas L, Guiu B, Chalaye J et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2020; Published Online: November 06, 2020 https://doi.org/10.1016/S2468-1253(20)30290-9
“Personalized dosimetry is safe and leads to a meaningful improvement in the objective response rate and overall survival of patients with locally advanced hepatocellular carcinoma [...] when compared with standard dosimetry.”
Study Objective and Design
A randomized, multicenter, investigator sponsored phase II trial comparing the clinical outcomes of SIRT with TheraSphere in patients with advanced HCC using two pre-treatment dosimetry determination methods: (1) Standard, single-compartment dosimetry (STD); defined as a uniform distribution of absorbed dose within the perfused volume – both tumor and normal liver or (2) Personalized dosimetry (PERSO); defined as multi-compartment Y90 distribution of absorbed dose within the perfused volume that accounts for preferential blood flow into the tumor compared with normal parenchyma.
Study Design

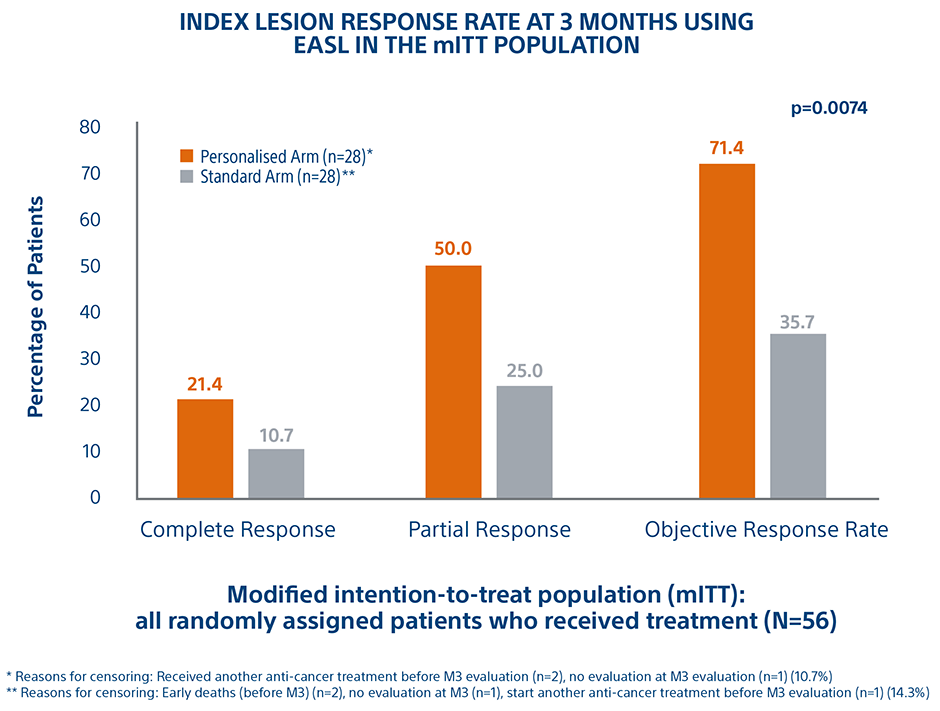
Personalized Dosimetry Improves Response
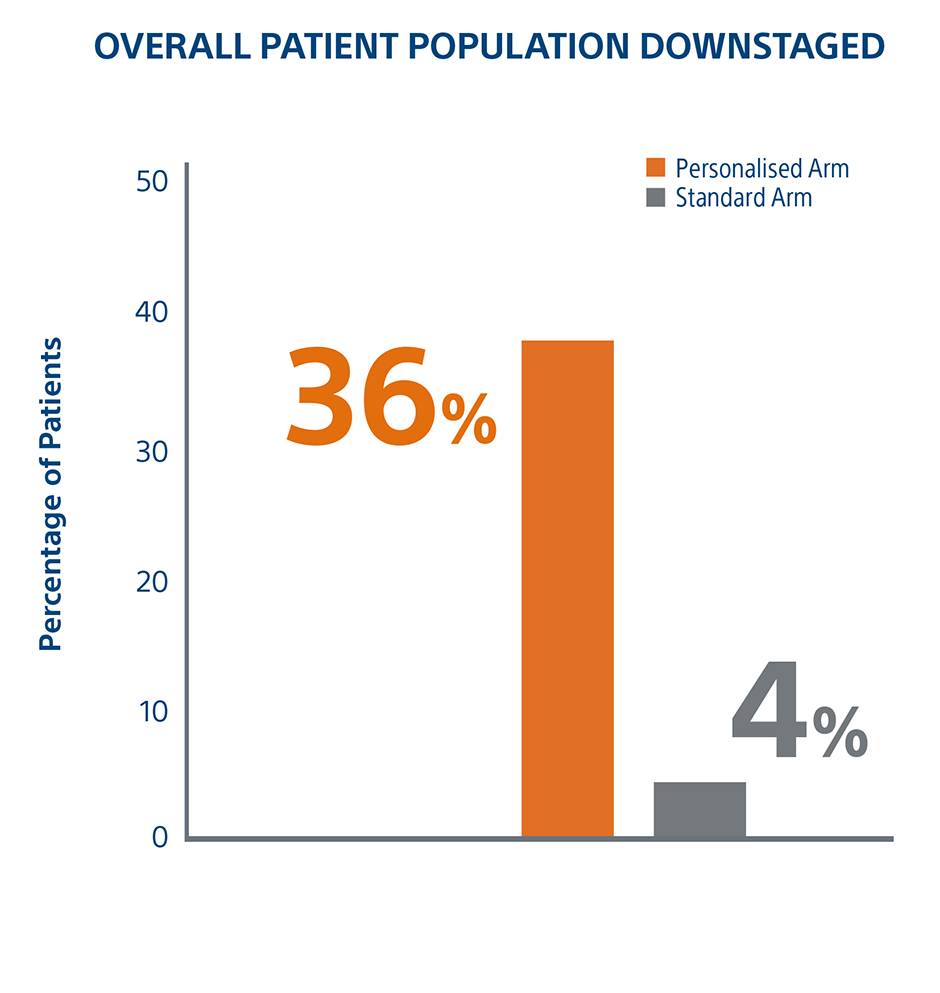
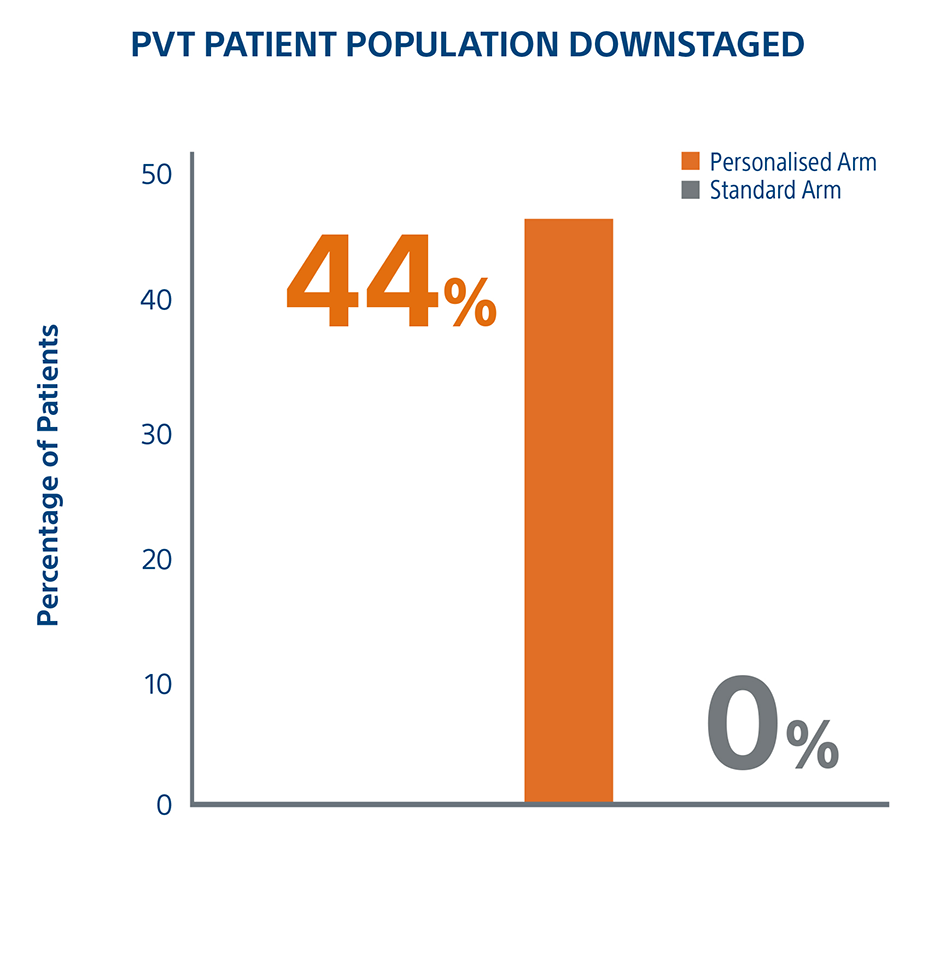
Personalized Dosimetry Downstages More Patients To Surgery
DOSISPHERE-01 EDITORIAL
“The DOSISPHERE-01 Study challenges the evolving narrative that patients with advanced hepatocellularcarcinoma should have systemic therapy at the expense of locoregional therapy. This notion is particularly true for patients with large tumours and local vascular invasion.”1
–Robert J Lewandowski, MD, Riad Salem, MD, DOSISPHERE Editorial, Lancet Gastroenterology & Hepatology
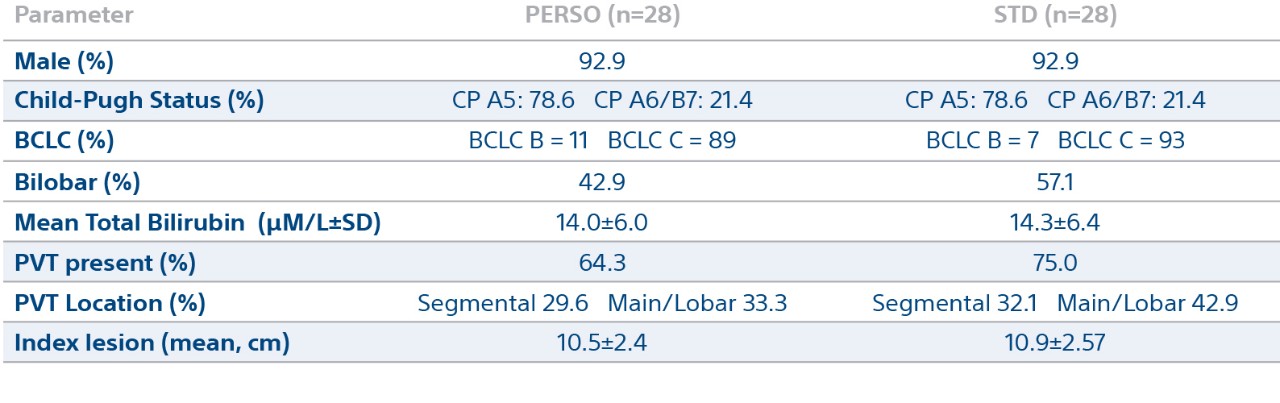
Patient Demographics
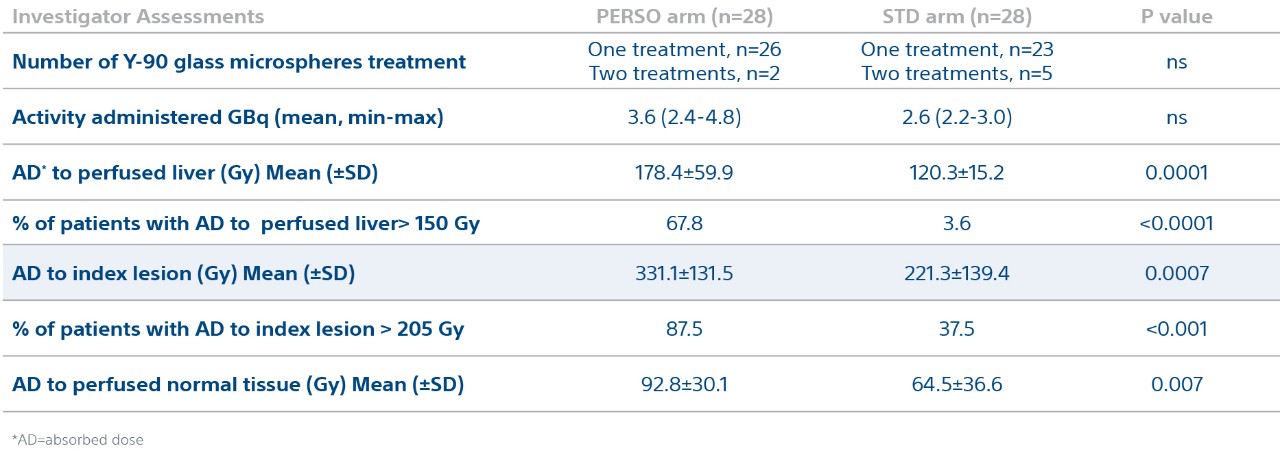
Treatment Characteristics and Dosimetry
Liver Adverse Events (Grade ≥3) Related to Y-90*
| PAD (n=35) | SDA (N=21) | |
| Patients with ≥ 1 AE | 3 (8.6%) | 3 (14.3%) |
| Death | 1 (2.9%) | 1 (4.8%) |
| Liver AEs | 4 (11.4%) | 5 (23.8%) |
| Ascites | 1 (2.9%) | 2 (9.5%) |
| Encephalopathy | 0 | 0 |
| GI hemorrhage | 0 | 2 (9.5%) |
| Bilirubin increase/jaundice | 1 (2.9%) | 2 (9.5%) |
| Hepatic failure | 2 (5.7%) | 0 |

Perform SIRT with TheraSphere™

SIMPLICIT90Y™
Embrace Y90
SIRT Personalised Dosimetry
SIRT Personalised Dosimetry

![]()
Virtual Events

![]()