1. TheraSphere Yttrium-90 Glass Microspheres – Instructions for Use – Rev 9.0.
2. Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J Vasc Interv Radiol. 2006;17:1251–78.
3. Pellerin O, Lin M, Bhagat N, et al. Can C-arm cone-beam CT detect a micro-embolic effect after TheraSphere radioembolization of neuroendocrine and carcinoid liver metastasis? Cancer Biother Radiopharm. 2013;28(6):459–65.
4. Atassi B, Bangash AK, Bahrani A, et al. Multimodality imaging following 90Y radioembolization: A comprehensive review and pictorial essay. Radiographics. 2008;28(1):81–99.
5. Young S., Chen T, Flanagan S, et al. Realized tumor to normal ratios in hepatocellular carcinoma patients undergoing transarterial radioembolization: A retrospective evaluation. Eur Radiol. 2022;32:4160-7.
6. Toskich B, Vidal LL, Olson MT, et al. Pathologic Response of Hepatocellular Carcinoma Treated with Yttrium-90 Glass Microsphere Radiation Segmentectomy Prior to Liver Transplantation: A Validation Study. J Vasc Interv Radiol. 2021;32(4):518-526.e1.
7. Montazeri SA, De la Garza-Ramos C, Lewis AR, et al. Hepatocellular carcinoma radiation segmentectomy treatment intensification prior to liver transplantation increases rates of complete pathologic necrosis: an explant analysis of 75 tumors. Eur J Nucl Med Mol Imaging. 2022;49(11):3892-3897.
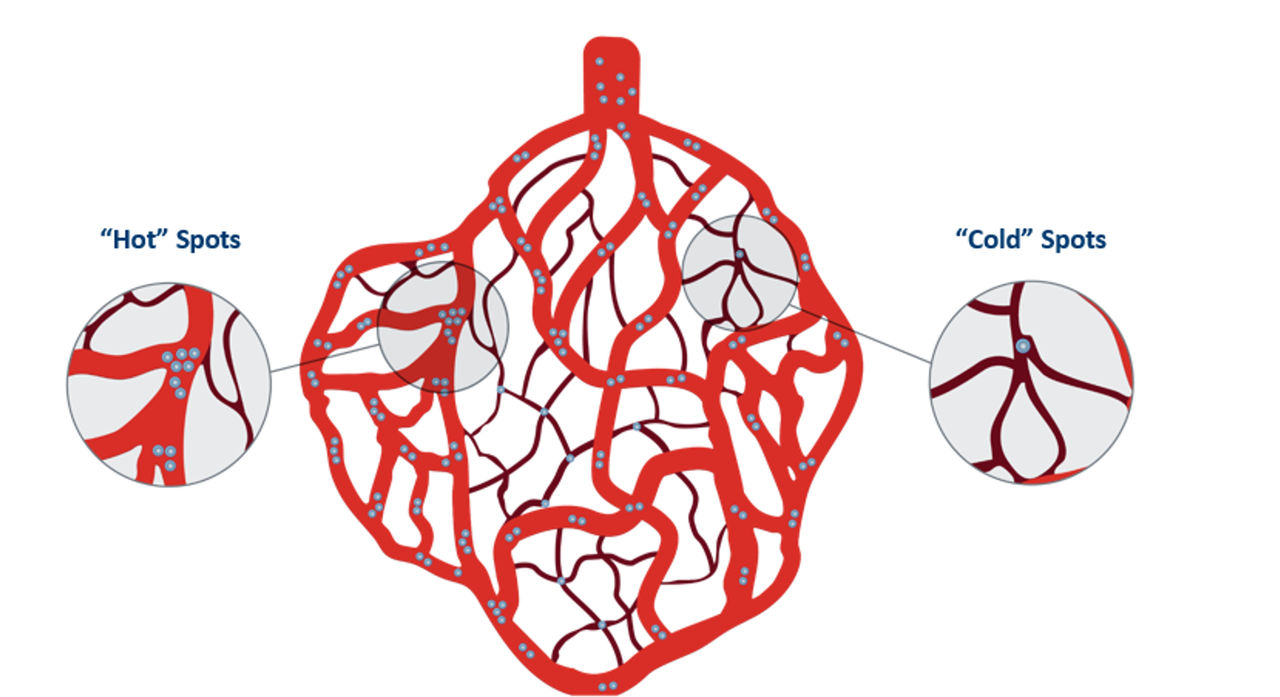
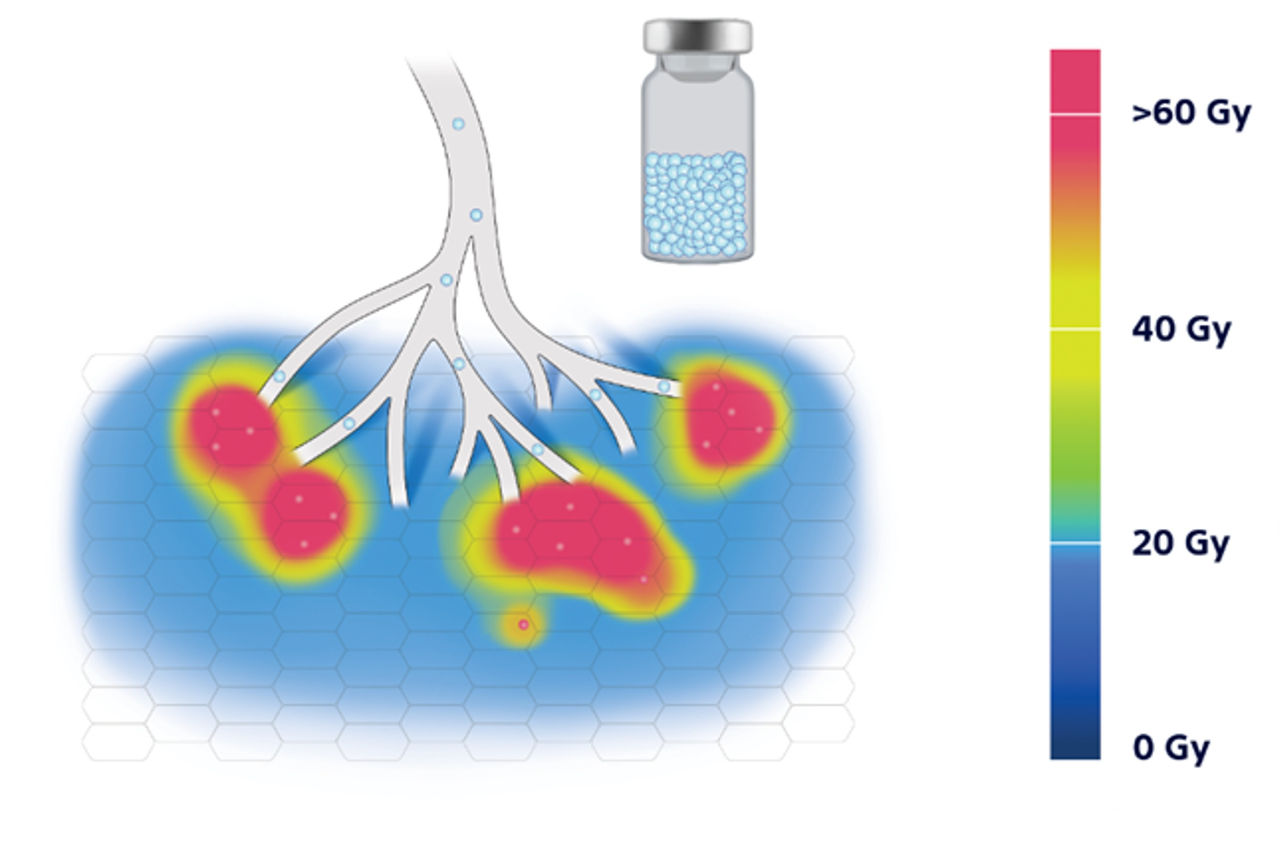
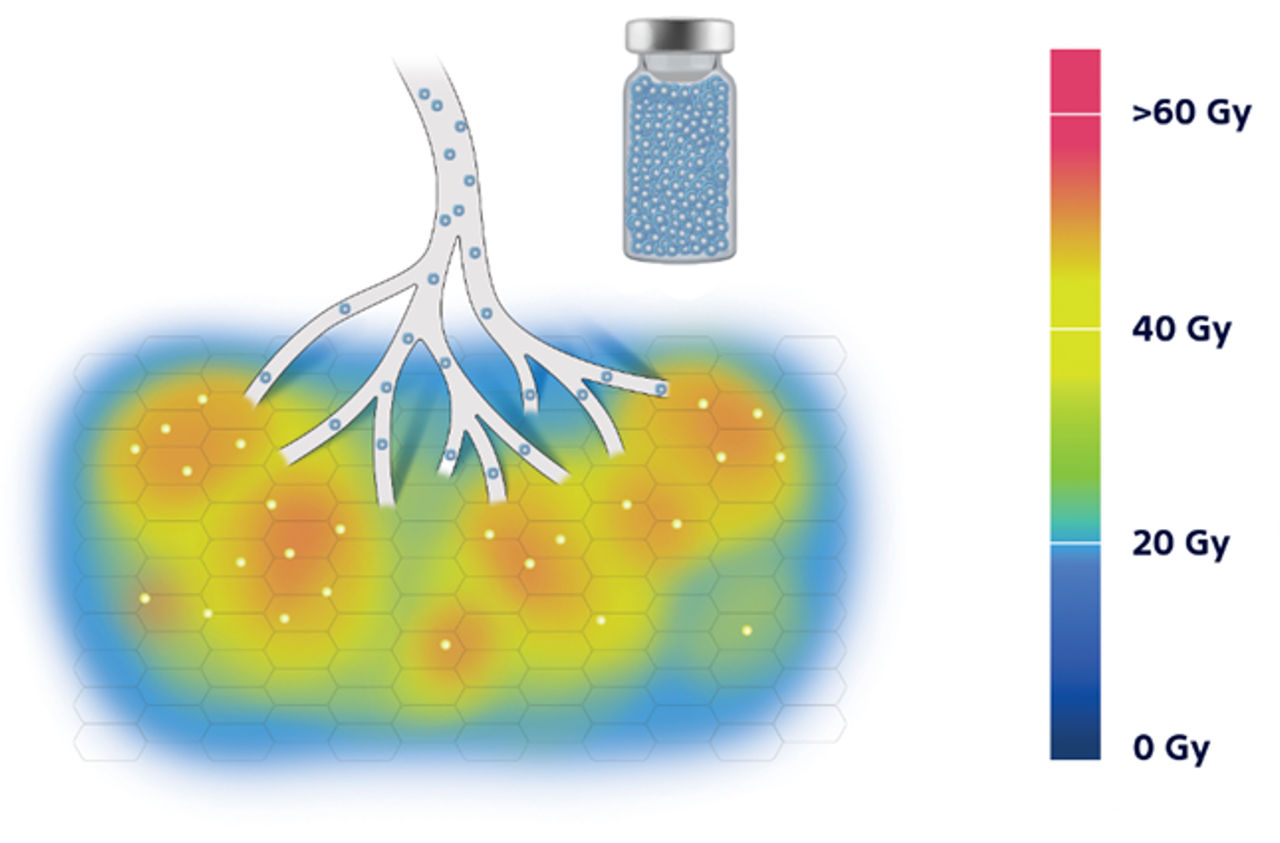
8. Pasciak, A. S., Abiola, G., Liddell, R. P., Crookston, N., Besharati, S., Donahue, D., Thompson, R. E., Frey, E., Anders, R. A., Dreher, M. R., & Weiss, C. R. (2019). The number of microspheres in Y90 radioembolization directly affects normal tissue radiation exposure. European Journal of Nuclear Medicine and Molecular Imaging, 47(4), 816–827. https://doi.org/10.1007/s00259-019-04588-x.
9. Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): A randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(1):17-29.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.