Setting the standards in liver cancer care
TheraSphere™ is supported by guidelines for effective HCC management1-4 and remains at the forefront of cancer treatment, continually adding to its robust body of scientific evidence.
Our commitment to improving liver cancer care is reflected by the +30,000 patients that have already been treated with TheraSphere™ Y-90 Therapy in Europe in the past two decades. We continue setting the standards in liver cancer care.
Transforming SIRT practice through clinical evidence
With +40 investigator-led studies supported by Boston Scientific (9 of which specifically focused on investigating combination IO therapies), we are continuously adding to our strength of evidence supporting the benefits of TheraSphere™ Y-90 Therapy, from bridging and downstaging to lobectomy and segmentectomy.
| 2016 | PREMIERE |
| Bridged to surgery. Reduced drop out from waiting list. | |
| 2020 | DOSISPHERE-01 |
| TheraSphere™ with personalised dosimetry improved objective response rate and overall survival. | |
| 2021 | LEGACY |
| Changed guidelines with the inclusion of SIRT in BCLC 2022 guidelines | |
| 2022 | TARGET |
| Real-world data confirmed that Dose Matters to Improve Survival | |
| 2022 | RASER |
| Established Radiation Segmentectomy for Curative Intent | |
| 2022 | TRACE |
| Improved outcomes with TheraSphereTM Y-90 vs. DEB-TACE | |
| 2024 | PROACTIF (Interim data, final publication coming this year) |
| The largest, prospective, real-world, Y-90 study of primary liver cancer. |
The versatile and only proven treatment endorsed by international guidelines
TheraSphere™ Y-90 research covers the full BCLC guidelines – from disease control to curative intent – driving the evolution of SIRT and setting the standards of liver cancer care.
Indication | BCLC1 | EASL2 | ESMO3 | ILTS-ILCA4 |
Alternative to TACE | ✔ | ✔ | ✔ | ✔ |
Bridging to Liver Transplant | ✔ | ✔ | ✔ | ✔ |
Bridging to Liver Resection | ✔ | ✔ |
| |
Downstaging to curative surgery | ✔ | ✔ |
| ✔ |
Radiation segmentectomy | ✔ | ✔ | ✔ |
|
Guidelines recommend SIRT as an alternative to TACE in selected patients2,3
- EASL Guidelines
- ESMO Guidelines
- Large HCC (>7 cm): Lower risk of adverse events (AEs)
- Intermediate stage: SIRT [II, B] can be considered as alternative to TACE
- Bridging to LT: If waiting list time > 3 months
- Bridging to LR: Radiation lobectomy for FLR increase while providing durable tumour control
- Downstaging to LT: Superior local tumour control. Better tumour shrinkage enabling LT in patients beyond Milan Criteria
- Small tumour: Superior over TACE for small tumour
- Single tumour ≤ 8 cm: An alternative option for patients who are unfavourable for resection (BCLC A)
- Intermediate stage HCC: An alternative to TACE
- Bridging to OLT: When prolonged waiting time (>3 months) anticipated
What differentiates TheraSphere™ Y-90 from TACE?
Understanding the differences between treatment options is crucial for better patient outcomes in liver cancer care. By offering a precise, radiation-based approach that directly targets liver tumour, TheraSphere™ Y-90 Therapy not only enhances tumour control but also improves patient quality of life by reducing side effects and extending progression-free survival.
Efficacy
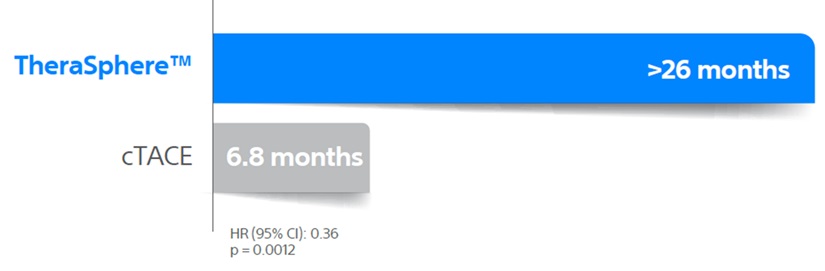
As demonstrated in the PREMIERE6 Trial, TheraSphere™ delivers superior tumour control over cTACE and longer Time To Progression (TTP), which could result in reduced liver transplant dropouts.
Median TTP
Safety
TheraSphere™ is a tolerable treatment with a better safety profile. Less procedures positively impact patient lives.5
Cost effectiveness
SIRT is a more cost effective downstaging strategy, with an incremental QALY gain vs. TACE of 0.22. Additionally, successful downstaging is achieved with only 2 sessions with SIRT while TACE required 3.7
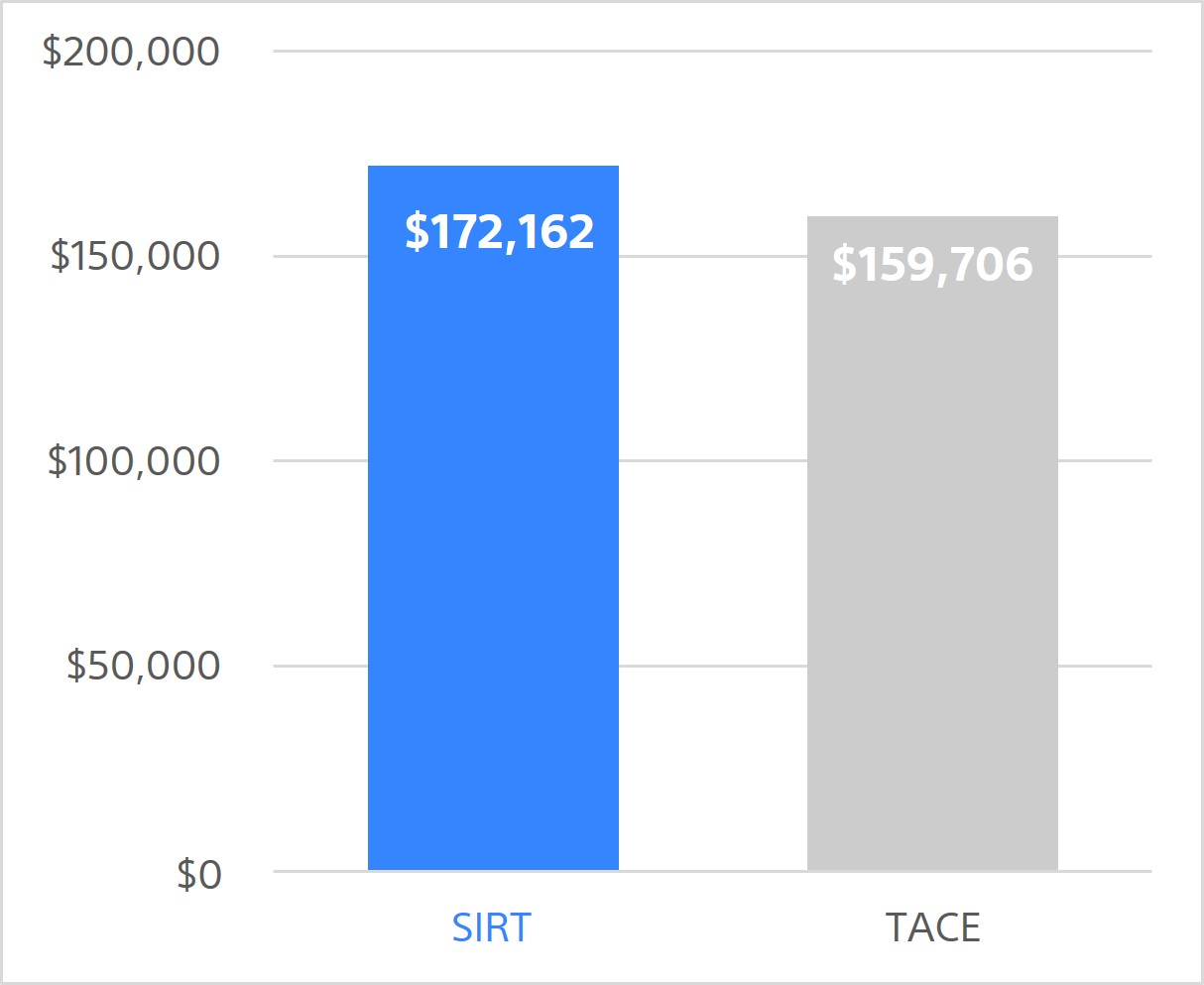
Total costs over 5 years
(per person)
Total QALYs over 5 years
(per person)
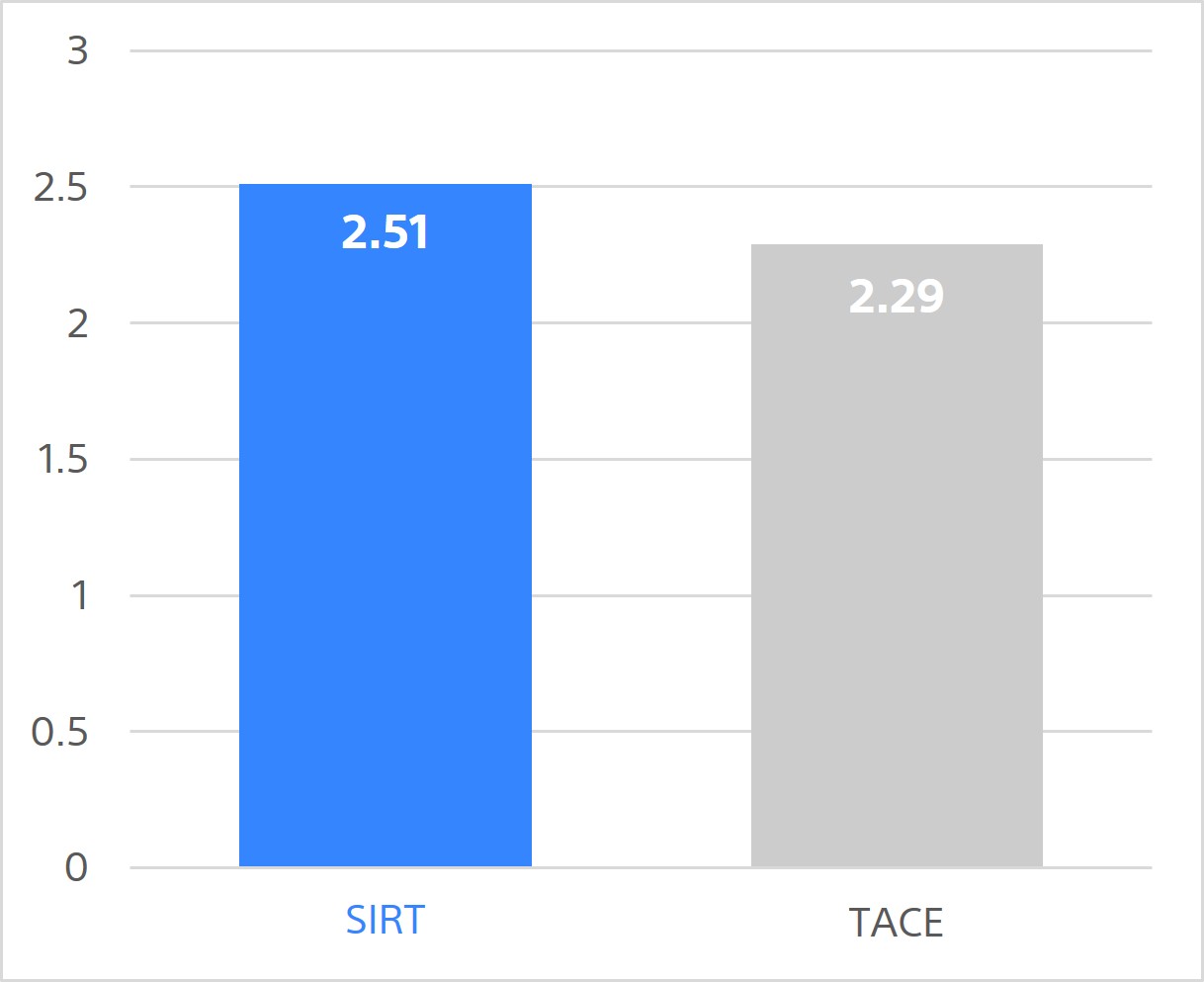
SIRT is associated with slightly higher overall 5-year costs. However, it's more effective, providing greater health gains, with an incremental QALY gain vs TACE of 0.22. That is equivalent to 80 days in perfect health or 104 days (nearly 3.5 mo) in compensated cirrhosis state.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
*Hepatocellular Carcinoma
1. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022; 76(3): 681-93.
2. EASL. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J Hepatol. 2025;82(2): 315-374.
3. Vogel A, Chan SL, Dawson LA, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2025;36(5):491-506.
4. Kodali S, Kulik L, D'Allessio A, et al. The 2024 ILTS-ILCA consensus recommendations for liver transplantation for HCC and intrahepatic cholangiocarcinoma. Liver Transpl. 2025;31(6):815-831.
5. Dhondt E, Lambert B, Hermie L, et al. 90Y Radioembolization versus Drug-eluting Bead Chemoembolization for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase Il Randomized Controlled Trial. Radiology. 2022;303(3): 699-710.
6. Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151(6):1155-63.e2
7. Wu X, Kwong A. Heller M, Lokken RP, Fidelman N, Mehta N. Cost-effectiveness analysis of interventional liver-directed therapies for downstaging of HCC before liver transplant. Liver Transpl. 2024;30(2): 151-159.
8. Rognoni C, Barcelona MR, Bargellini I et al. Cost-effectiveness analysis of personalised versus standard dosimetry for selective internal radiation therapy with TheraSphere™ in patients with hepatocellular carcinoma. Front Oncol. 2022;12: 920073.