Enrollment Begins for Novel Trial Studying PCI in Underserved Populations
Dr. Craig Thompson interviews Dr. Roxana Mehran about the PLATINUM Diversity Trial. Dr. Mehran is a Co-Principal Investigator of the PLATINUM Diversity Trial and Professor of Medicine and Clinical Science at the Icahn School of Medicine at Mount Sinai Hospital in New York and a leading advocate for the diversification of clinical science.

PLATINUM Diversity Addresses an Unmet Clinical Need in PCI Clinical Trials
More Information
Close the Gap is Boston Scientific's health equity initiative, which aims to eliminate cardiovascular care disparities. Learn more
Interview Video Transcript
Dr. Thompson: Hello, I’m Craig Thompson, Chief Medical Office of Interventional Cardiology for Boston Scientific. I’m at the American Heart Association 2014 Annual Scientific Sessions, joined here today by Dr. Roxana Mehran.
Dr. Mehran is Professor of Medicine and Professor of Clinical Science and economics at the Icahn School of Medicine at Mt. Sinai Hospital in New York, world-renowned Interventional Cardiologist and investigator. Dr. Mehran is principal investigator along with a co-principal investigator Dr. Wayne Batchlor of Tallahassee, of the PLATINUM Diversity Clinical Trial.
Roxana, welcome.
Dr. Mehran: Thank you for having me, Craig.
Dr. Thompson: We’re here today to discuss the PLATINUM Diversity. We’re evaluating outcomes in patients in a real world setting but that’s not all that’s unique about this trial, is it Roxana?
Dr. Mehran: First of all, thank you for having me. How fitting to be here at the American Heart Association, the association that's committed to health care delivery and cardiovascular health in all men and women, black or white, and of all racial backgrounds, and I think what an incredible honor for me to lead the Diversity trial.
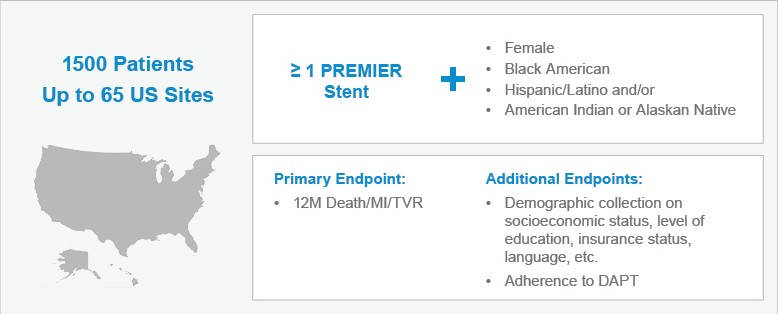
The PLATINUM Diversity study as you know is a 1,500-patient study, a single-arm study, I like to call it, that's going to look at outcomes of patients who are usually underrepresented in clinical trials. Who are those patients? They're women, they're African Americans, and they're of Latina backgrounds, and Hispanic Americans, and I think this is an incredibly important study because the health outcomes of these patients are often unknown.
Why? Because they're underrepresented in most of the trials, encompassing less than 30% of the trial population, and as such, I've always been concerned whether or not the therapies that we currently have available in the armamentarium of cardiovascular health is actually applicable to this population. We all know that there are so many important issues for these patients that we need to deal with, whether it's cultural, whether it's access to health, whether it's making sure that they reach their goals, and they're compliant and adherent to their medications and discharge instructions.
All of those things are part and parcel of what we do as we deliver health care to all of our patients, not just to the diverse group. I'm extremely excited and I want to congratulate Boston Scientific for actually taking this step forward and closing the gap, and I believe that there is a gap in knowledge, of understanding how to best care for these patients, and I think through this trial, we're going to learn a tremendous amount.
Dr. Thompson: Roxanna, how are treatment plans currently formulated in patient populations where there is little or no clinical data that’s been generated?
Dr. Mehran: The current treatment plans of how we apply the evidence base, even from everything we've learned here at this congress and how do we bring it home, is very much unified and we apply it across the board and I'm not so sure that that's the right way to do this.
That's yet another reason why I think it's important for us to deeply understand who these people are, what are the special issues for them and how can we best be able to deliver those important health care messages and delivery of care.
Dr. Thompson: How does this data set, how would you envision that this data set can help you advance your advocacy efforts within societies and other interested bodies to move the field forward?
Dr. Mehran: It's a really, really important and pointed question. I think we can look at this and for me, to have a data set that is enriched with women is just so exciting, to be able to better understand what are some of the important gender issues and then to say that I'm actually going to have data on African American women, I'm really thrilled about that.
That's just going to be something that we just never had and I think we're very excited about that part … and Hispanic women, we want to learn about, very importantly, about the risk factors that are in these patients, especially diabetes, hypertension, hypercholesterolemia, and those are going to be very much collected in this trial. We're going to link a lot of this and understand best how to care for these patients.
It's important to note that the Association for Black Cardiologists are very much behind this initiative and I think I'm really proud and I'm so thankful for their leadership to really stand behind this trial and really, really help us.
And then very importantly, Women in Cardiology, the WIN, SCAI-WIN group, is so very, very excited and behind this very important initiative as well.
I just think that when we think about these types of trials dedicated to patients who are underrepresented in clinical trials, we have to really use the opportunity and seize the moment and get as much information as we can to improve their health outcomes, and we really are very, very grateful that Boston Scientific has taken the very first step forward towards this goal.

















