An interventional cardiologist and a vascular surgeon’s perspective on the unique features of the Lotus™ Valve, the REPRISE III trial, and the future of TAVR.
Interview with REPRISE III Principal Investigators
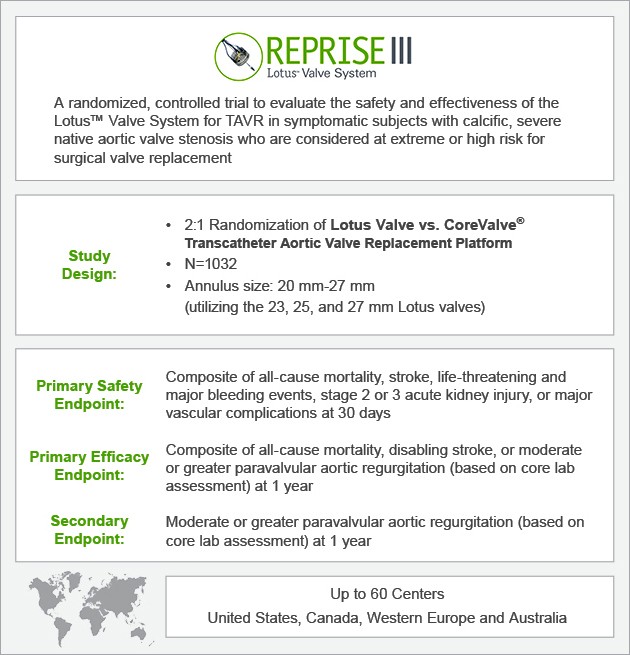
“What’s different about the REPRISE III trial design?”
“In ten years, what proportion of patients with aortic stenosis will still be treated surgically?”
“What patient needs and anatomical considerations are being covered by REPRISE III?”
“What are your thoughts about alternative access?”
“Why was CoreValve chosen as the comparator for REPRISE III?”
The Lotus Transcatheter Aortic Valve Design Goals
- Innovative adaptive seal designed to mitigate paravalvular insufficiency
- Fully repositionable from final locked position prior to release
- Early valve function to minimize hemodynamic disruption
REPRISE III Trial Design
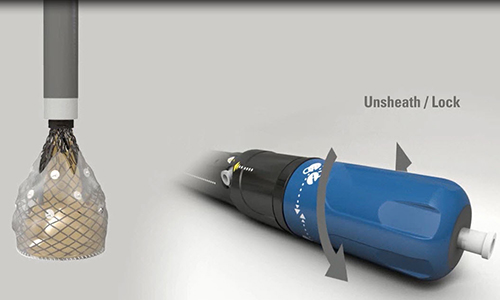
Lotus Deployment Mechanism
Interview Transcript
Keith: Hello, ladies and gentlemen, thank you for joining us today. It's Keith Dawkins here. I'm the Chief Medical Officer at Boston Scientific, and I'm pleased to have with me the two PIs of REPRISE III, the pivotal IDE trial of the Lotus Valve: Ted Feldman from Evanston Hospital in Chicago and Mike Reardon, Cardiac Surgeon, Chief of Cardiac Surgery from Houston Methodist. Over the next few minutes, Ted and Mike are going to answer some questions around this really exciting trial, REPRISE III.
The first question for you, Ted: What features of Lotus Valve do you think differentiate it as a novel TAVR device, compared with the other available devices in the US?
Ted: The major differentiator is in terms of what we're already seeing in outcomes and the initial experience, and that is a virtual elimination of paravalvular leak as a limiting factor in the TAVR procedure. The other differentiators are procedural. From an operator's standpoint, this is the most controllable and least precipitous of the TAVR procedures that any of us have been involved with. Part of the advantage in outcomes relates to the ability to very precisely position, reposition, or completely retrieve the device. These are all truly unique.
Keith: Great. Thanks. Obviously, in the REPRISE II CE Mark trial, it was obvious from the results, and the REPRISE II extension trial, that those features have been translated into great outcomes.
Now moving across to you, Mike. You're obviously an experienced operator in a number of large valve trials. Tell us about REPRISE III, and what's new with this trial design.
Mike: It's a very exciting trial, because it is the first trial to look at the really new generation TAVR valves. What's new about this is it's going to randomize against a commercially available valve in the United States, which is something new for us to do. I think it's going to allow us to collect a large number of data points on this new valve and compare it against what we're using commercially in the United States right now, so it's a very exciting trial design.
Keith: Obviously, this is a randomized trial design. Again, two valves, one against another, whereas, previously, designs tended to compare TAVR with surgery. As a surgeon, do you feel comfortable with this sort of trial design?
Mike: Absolutely. Right now, this trial includes extreme risk, which I would not operate on anyway, and high risk, where I think the data is very much in favor of TAVR these days. I think this is a great trial design, and it will allow us to give what is what I consider the newest generation valve on our hands, and compare it to what we're using on a day-to-day, commercial basis.
Keith: Let me ask you an unscripted, provocative question, Mike. If we're having this conversation in ten years' time, what proportion of patients do you think with aortic stenosis will still be treated surgically?
Mike: Let me change your question a little bit. What proportion of patients that would be a candidate for a tissue valve would be treated surgically? I think less than 20%. I think the major issue right now left, there's two. One is paravalvular leak, because we know we do hemodynamics; we know stroke is OK; we know morbidity is as good as surgery, but paravalvular leak and durability. I think this valve design is going to bring paravalvular leak in line with surgical valves, and only time will tell us about durability.
Keith: Thanks.
Moving to you, Ted. Obviously, there are patients that have been included and excluded in this trial. What patient needs and anatomical considerations are being covered by REPRISE III, with the Lotus Valve?
Ted: Currently, the defining anatomical features to include patients for treatment in REPRISE III are the annulus diameter. We have three sizes of valve diameters: 23, 25, and 27 mm that'll treat a range of valve annulus diameters from 20 to 27 mm, and getting below or above that range is going to require additional valve sizes. The other feature is femoral access, and, currently, our 23 valve uses an 18-French-compatible system, and the 25 and 27 are a closer to 20-French system that requires about a 6.5 mm iliofemoral vessel. I think the Lotus development team has already been very active at developing next-generation Lotus delivery systems and valves. We're going to see both the annulus and femoral access limitations develop very steadily.
Keith: Absolutely. The 21 mm and the 29 mm are already in development, as is the next-generation delivery system, when we'll look at reducing the French size.
That actually moves us nicely on, Mike, to thoughts about alternate access for TAVR. Clearly, initially, there was quite a lot of interest in the transapical approach, particularly with the larger devices, first-generation devices. Now it's increasing in interest from surgeons around direct aortic and subclavian. What are your thoughts around alternative access?
Mike: If we look at our comparator valve, which is the CoreValve, in its IDE trial, 80% of the cases could be done femorally, and it has a delivery system that's very comparable to the Lotus delivery system. So I would expect that 80% of the patients we see will have adequate femoral arteries for access. But that still leaves 20% of the patients that we could not treat without an alternative access, with this particular platform. So I think that the size, expanding the annular range, expanding the access options is something that will give more patients access to this in the future.
Keith: In your practice, where a valve can be used either transapically or by the direct aortic route, What are your thoughts? Are you evolving more towards direct aortic?
Mike: I have always been evolved towards direct aortic, and I have to admit my bias there. I think that if your choice is to put a hole in your aorta or a hole in your heart and it's comparable at all other ways, that that's a much better way to go. As you bring in newer surgeons, your newer heart surgeons cannulate the aorta every day. Very few of them put something in the apex of the heart every day. I think as we evolve this therapy out to the broader population, direct aortic is going to be a more comfortable, likely safer way to go. I think there's some evolving data that with the transapical, there is some ventricular price to pay. Bill Fearon's work shows that the EF doesn't do as well as transfemoral. I think, with the direct aortic, from the ventricular standpoint, it will be no different than femoral.
Keith: I think patients, given the choice, probably prefer it post-procedure, don't they? There's probably less discomfort from a direct aortic.
Mike: Right. I think a mini sternotamy hurts a whole lot less than any thoracotomy. Thoracotomies are fairly uncomfortable. Of course, what all patients prefer is transfemoral, if we can do it.
Ted: I have to completely agree. The transaortic is going to be our next frontier, and I'd say for niche patients, we have several other alternative access procedures developing. One that I think is most interesting and not generalizable is transcaval, where venous access will be used to gain entry, ultimately, into the arterial system.
Mike: We also have a direct aortic system with a suprasternal approach, where I don't open your chest at all. So there are some very interesting things on the horizon. It will help us expand this platform to a greater number of patients.
Keith: Great. Final question for Ted. This, obviously, is a randomized IDE trial, valve against valve. There were a number of potential competitor valves. Why was CoreValve chosen as the comparative device?
Ted: If we were to do this randomized pivotal trial in Europe tomorrow, we'd have a dozen valves to have to sort through to find a comparator. We had the opposite problem in the United States, as we were nearing the point of launching this trial. CoreValve was the only approved platform that we really could use as a comparator, and that really drove the choice more than anything else.
Mike: Only Sapien was available, and I remember the Sapien introducer sizes were much larger. XT was not available, and we really needed something that had comparable delivery characteristics.
Keith: Yes. I think, just to clarify, this trial is predominantly a US-centered trial, but there are sites in a number of other countries, including Canada, Europe, and Australia so it was important to have a common device. Finally, of course, CoreValve had been shown in a randomized trial, to be superior to surgery in some patients, and so that, again, seemed a logical high bar for us to compare Lotus with.
I would like to thank the two of you. You're both very busy people. REPRISE III is nicely on track. You've both recruited patients to the REPRISE III, and we're very excited about your leadership for the trial. Thank you so much for joining me today, and we'll now sign off.