Dr. Reddy and Dr. Holmes Comment on the Recent WATCHMAN™ Post-FDA Approval, Initial US Clinical Experience Presented at TCT 2016
What is the Impact of These Data from the Perspective of an EP and an IC?
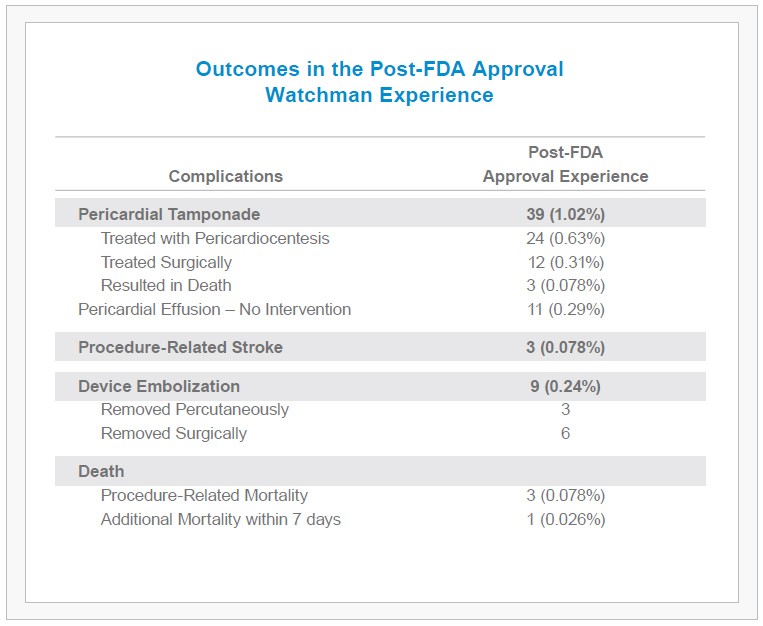
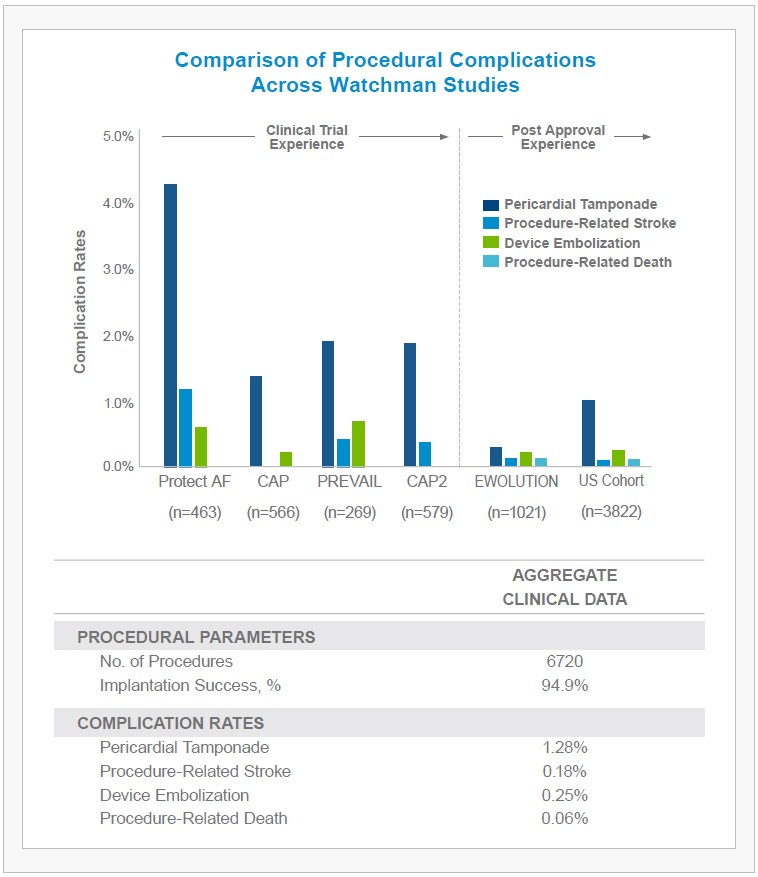
- 1.02% of patients experienced a pericardial tamponade (2/3 of which were treated percutaneously, without the need for surgery).
- An additional 0.29% of patients demonstrated pericardial effusion without hemodynamic effect, requiring no intervention.
- Device embolization, procedure-related stroke, and mortality rates also remained low at 0.24%, 0.08% and 0.08%, respectively




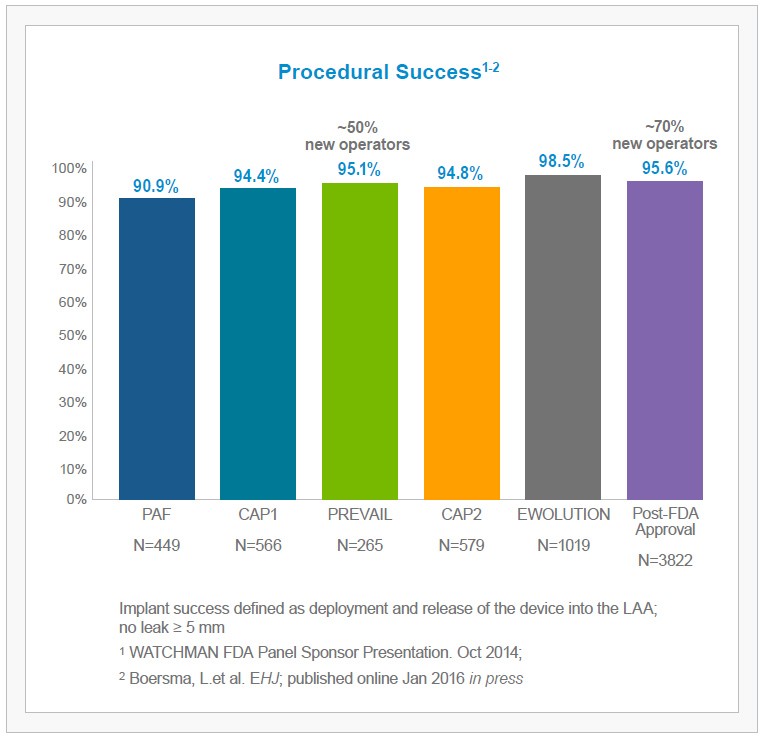
The WATCHMAN Experience in Perspective
Boston Scientific Resources
If you have a patient who may be a candidate for receiving the WATCHMAN Implant, use this tool to locate an implanting site in your area.
Interested in Performing Left-Atrial Procedures?
WATCHMAN implanters are highly experienced in transseptal crossing with training from advanced atrial fibrillation and structural heart programs, and all implanters complete a detailed training program specific to the WATCHMAN Implant. Contact your Boston Scientific representative to learn more.
Interested in Foundational Transseptal Access Training?
Boston Scientific now offers the first Transseptal Access Training Course for Interventional Cardiologists. This course provides a day of hands-on training for ICs experienced in structural heart, but looking to add transseptal puncture and left atrial access to their practice. The course covers imaging, 3D anatomy, equipment, basic technique, and complications relevant to transseptal access.
Note: This course is intended to provide foundational training prior to transseptal puncture credentialing and is not a substitute for site-specific certification or for WATCHMAN training (see below).
Interested in Learning More about Left Atrial Appendage Therapy with WATCHMAN?
Hear expert faculty discuss Left Atrial Appendage Closure (LAAC) as a treatment option for patients with NVAF, including the supporting clinical data, their experience with WATCHMAN, appropriate patient selection, and shared decision-making during WATCHMAN-focused, Boston Scientific-sponsored programs.
Contact your Local Sales Rep for More Information on Programs in your Area















