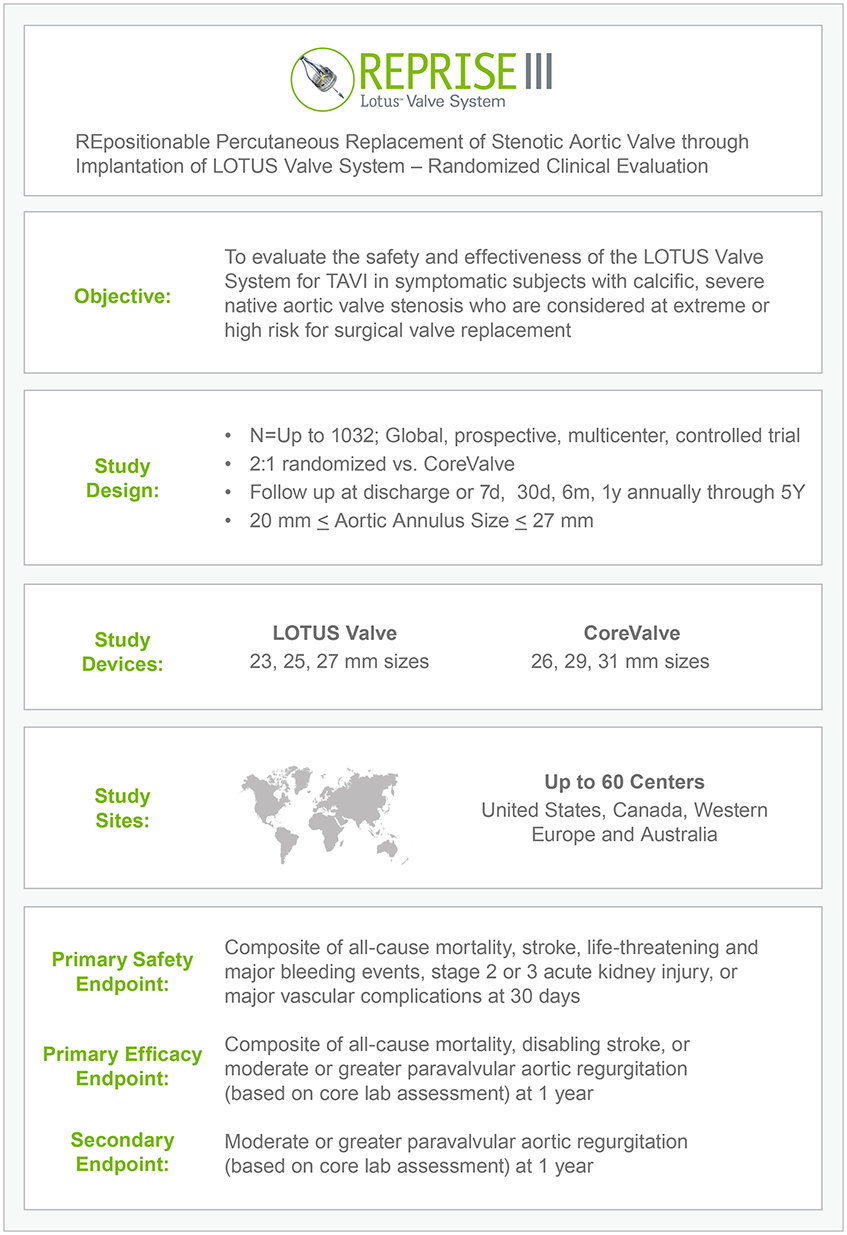
REPRISE III Completes Enrollment
First US Head-to-Head Lotus Valve vs. CoreValve Study Update
REPRISE III PI’s Dr. Feldman and Dr. Reardon Share Insight on Recent and Upcoming Milestones for the Lotus Valve
View Recent Lotus Valve Clinical Data Presentations