NAVIGATE-PF trial
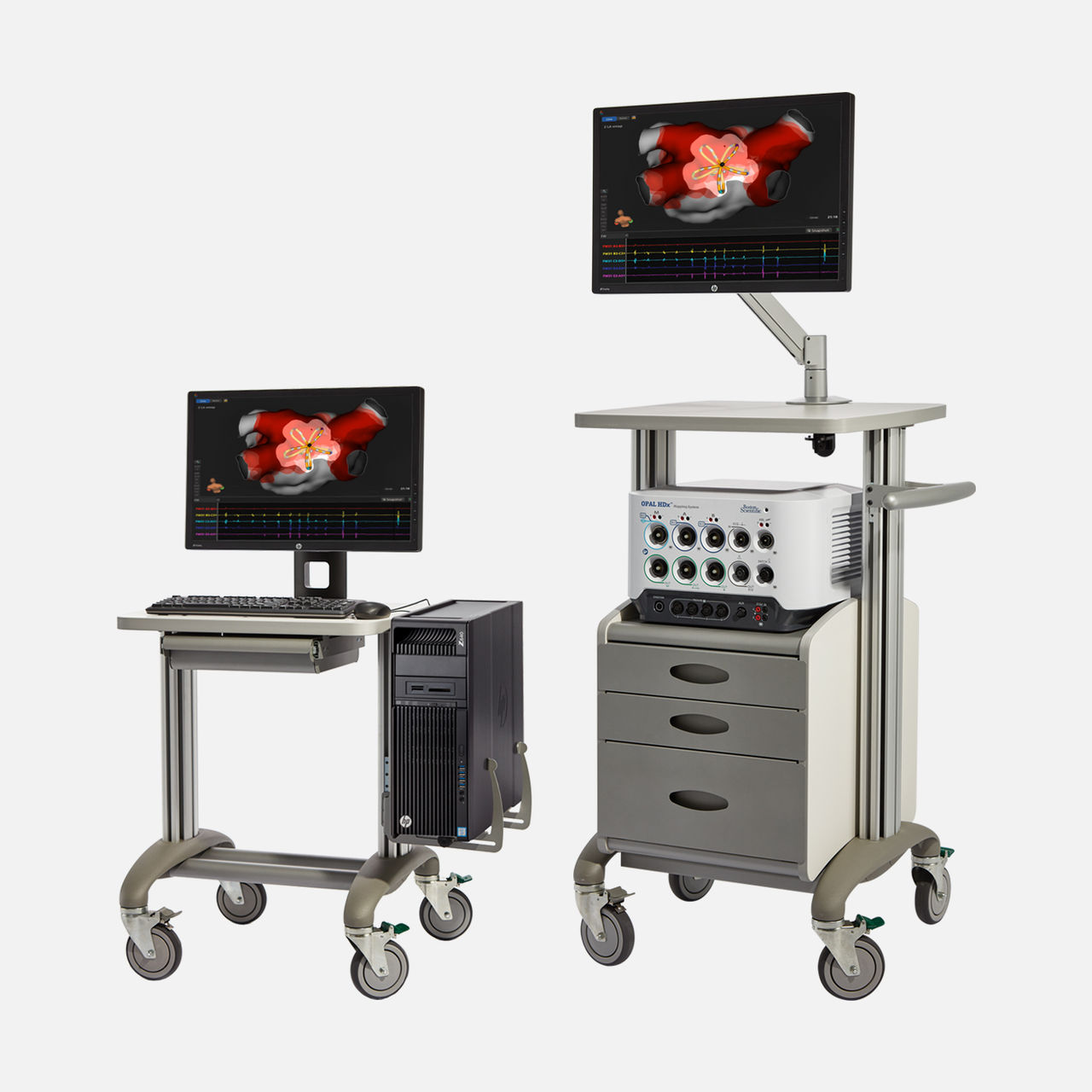
The NAVIGATE-PF clinical trial was a multi-center, first-in-human feasibility study that assessed the acute and chronic accuracy of FIELDTAG™ Technology on the FARAVIEW™ Software Module in visualising pulsed field energy delivery by the FARAWAVE™ NAV Pulsed Field Ablation (PFA) Catheter. The study included two phases:
Phase I (acute assessment):
- Phase 1 included 30 patients that underwent PV isolation, with 30% also receiving posterior wall ablation.PFA markers were placed during PFA application and high-quality, post-ablation HD maps with INTELLAMAP ORION™ Mapping Catheter were completed in 15 pts. PFA markers were overlaid on the HD map and contours were drawn at the outer border of the low voltage areas (defined as ≤0.5mV) where at least 2 overlapping PFA markers were dropped.
Phase II (chronic assessment):
- Included 20 patients at 1 center to evaluate chronic lesion durability and alignment between acute PFA tag locations and chronic low voltage borders.