IMPERIAL Clinical Trial Results
The IMPERIAL RCT demonstrated that Eluvia DES is clinically effective and safe in treating patients with symptomatic SFA disease, both in the short-term during the height of restenosis risk, and long-term out to five years.
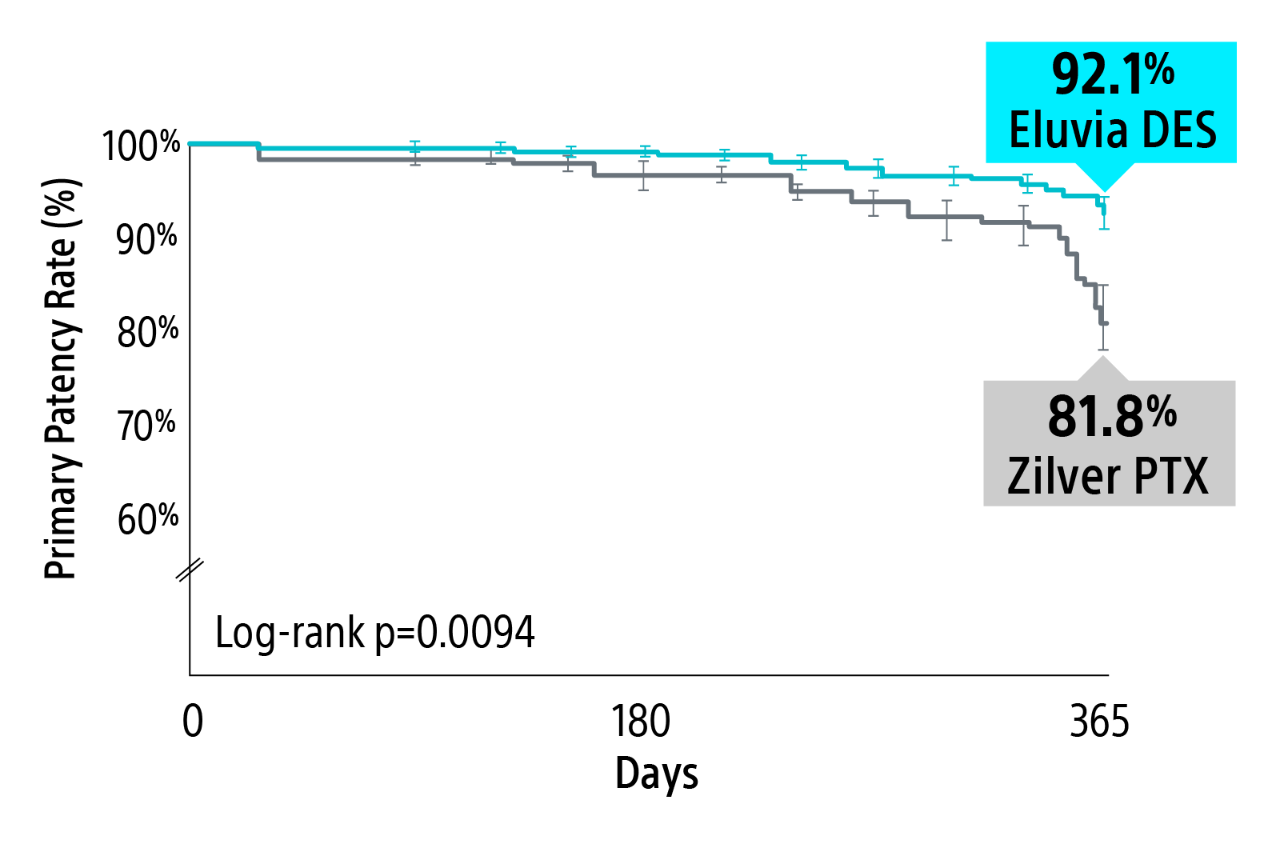
IMPERIAL RCT 1-year primary patency results
Eluvia Drug-eluting stent demonstrated superiority over Zilver PTX with a statistically significant primary patency through 1-Year.1
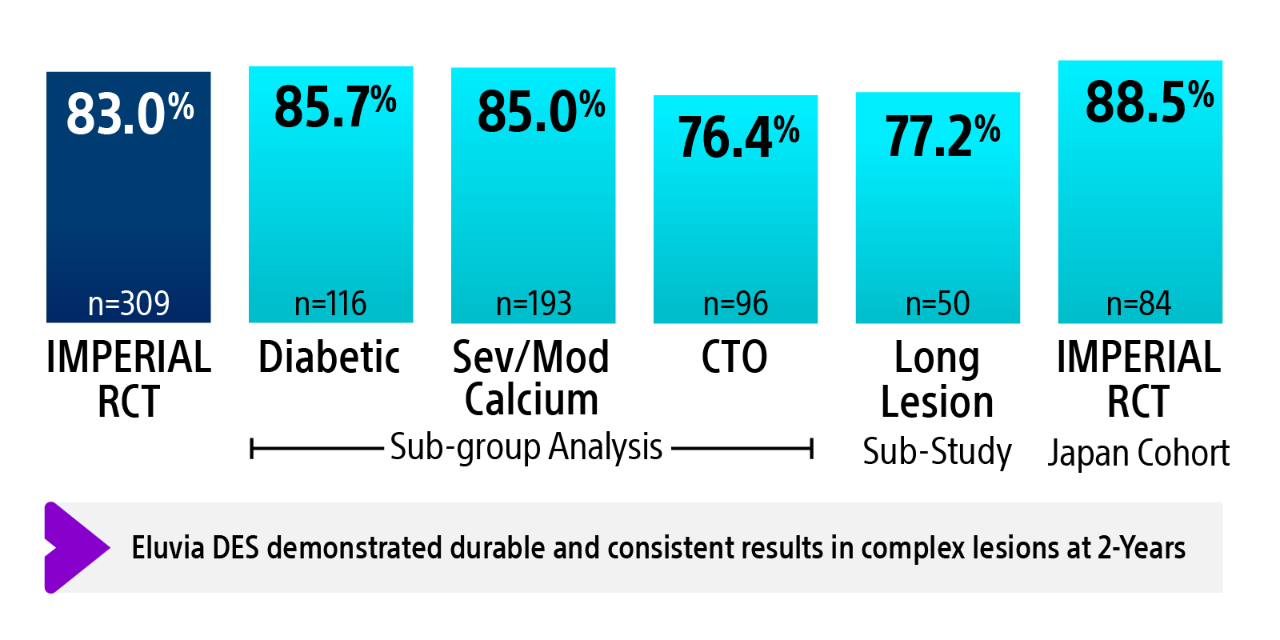
IMPERIAL RCT 2-year primary patency results2-6
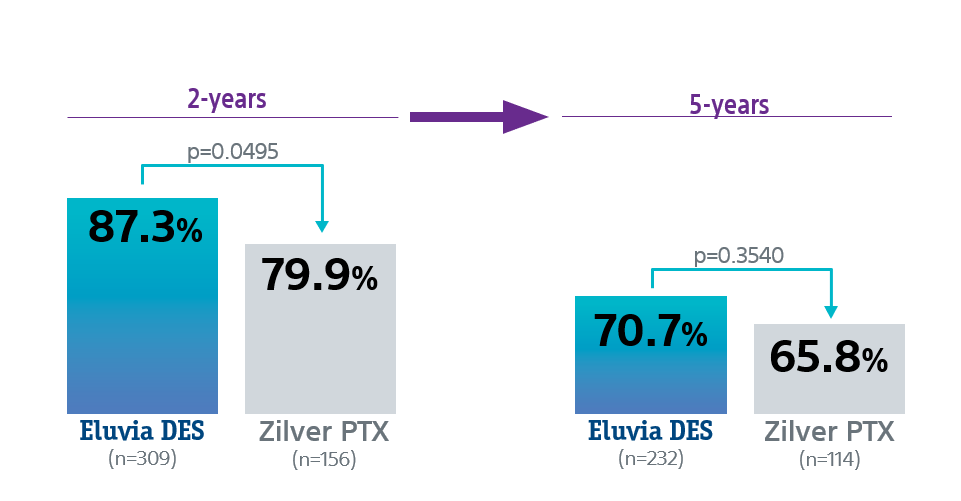
Freedom from CDTLR rates
Eluvia DES showed lower revascularization rates than Zilver PTX through 5 years with statistical significance at 2-Years.
IMPERIAL randomised controlled trial details
- 2-year primary endpoints
- Mortality rates
- Baseline characteristics
| 2-Year Primary Endpoints | Eluvia DES (n=309) | Zilver PTX (n=156) | p-value |
| Primary Patency | 83.0% | 77.1% | 0.1008 |
| Major Adverse Events | 14.2% | 20.1% | 0.1236 |
| Mortality Rates | Eluvia DES | Zilver PTX | p-value |
|---|---|---|---|
| 1-Year All-Cause Mortality | 2.1% | 4.0% | 0.23 |
| 2-Year All-Cause Mortality | 7.1% (21/295) | 8.3% (12/145) | 0.6649 |
| Baseline Characteristics | Eluvia DES | Zilver PTX |
| Age (Years) | 68.5 ± 9.5 | 67.8 ± 9.4 |
| Male Gender | 66.0% | 66.7% |
| Diabetes Mellitus | 41.7% | 43.6% |
| History of Smoking | 86.1% | 84.0% |
| Target Lesion Length (mm) | 86.5 ± 36.9 | 81.8 ± 37.3 |
| Severely Calcified | 40.1% | 32.3% |
| Total Occlusions | 31.2% | 30.3% |
| Extending into Distal SFA | 66.3% | 65.4% |