BHF PROTECT - TAVI
With stroke being #1 fear of TAVI patients –greater than death- while remaining unpredictable & devastating for patients, Boston Scientific actively supports the generation of new clinical data.
BHF PROTECT TAVI is a UK-based research study aiming to recruit 7730 participants undergoing TAVI* for treating severe aortic stenosis. The goal of the study is to demonstrate whether the use of CEP during TAVI impacts the risk of stroke.1
Watch below Dr. Tessel Vossenberg interviewing Prof. Rajesh Kharbanda for a comprehensive overview of the on-going BHF PROTECT TAVI trial and Cerebral Embolic Protection overall.
Study Design Overview
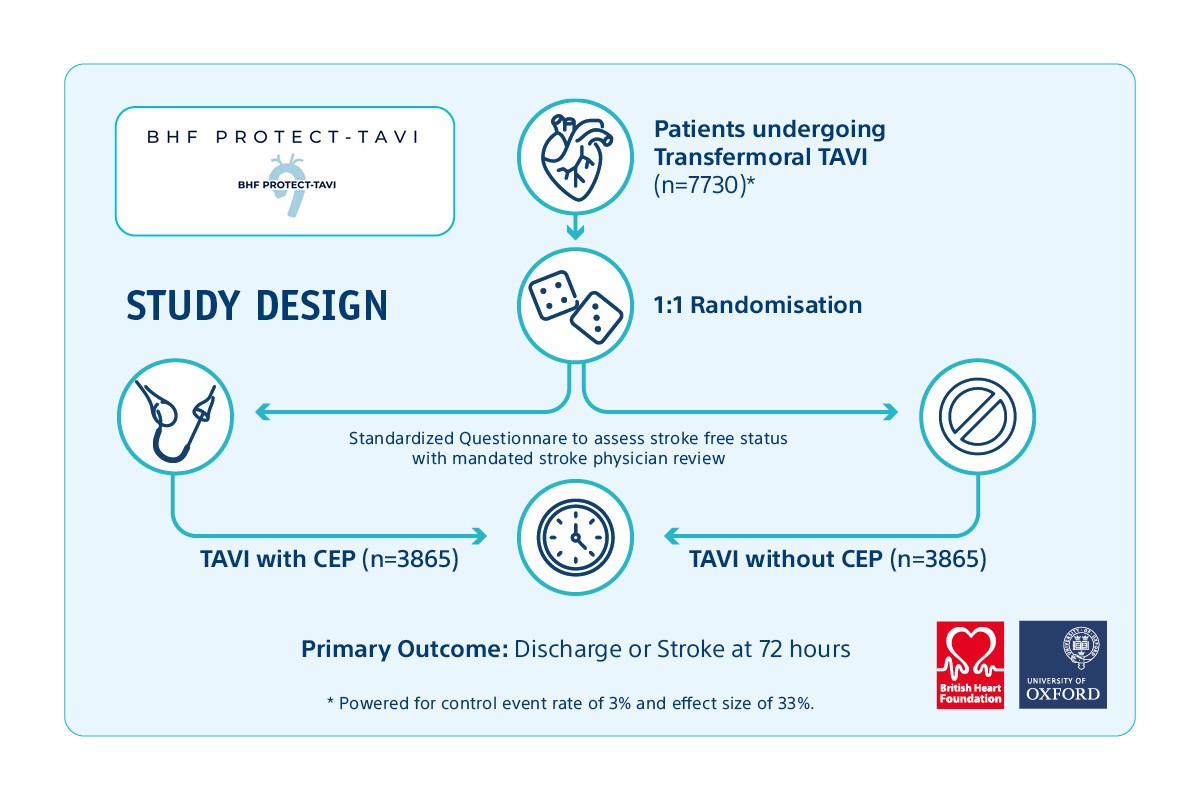
Boston scientific leads the way in the CEP field by actively supporting the BHF PROTECT TAVI study.
Here are some milestones for the study
Study commencement: June 2020
- 50% of enrollment & interim analysis: June 2023
- 70% of enrollment & interim analysis
- Enrollment completion: Q1 2025
- Data Release: 2025
Boston Scientific is leading the way by providing meaningful clinical evidence for Cerebral Embolic Protection
The PROTECTED TAVR trial
Study Objectives
To demonstrate that use of the SENTINEL™ CPS significantly reduces the risk of peri-procedural stroke (≤ 72 hours) after TAVR.
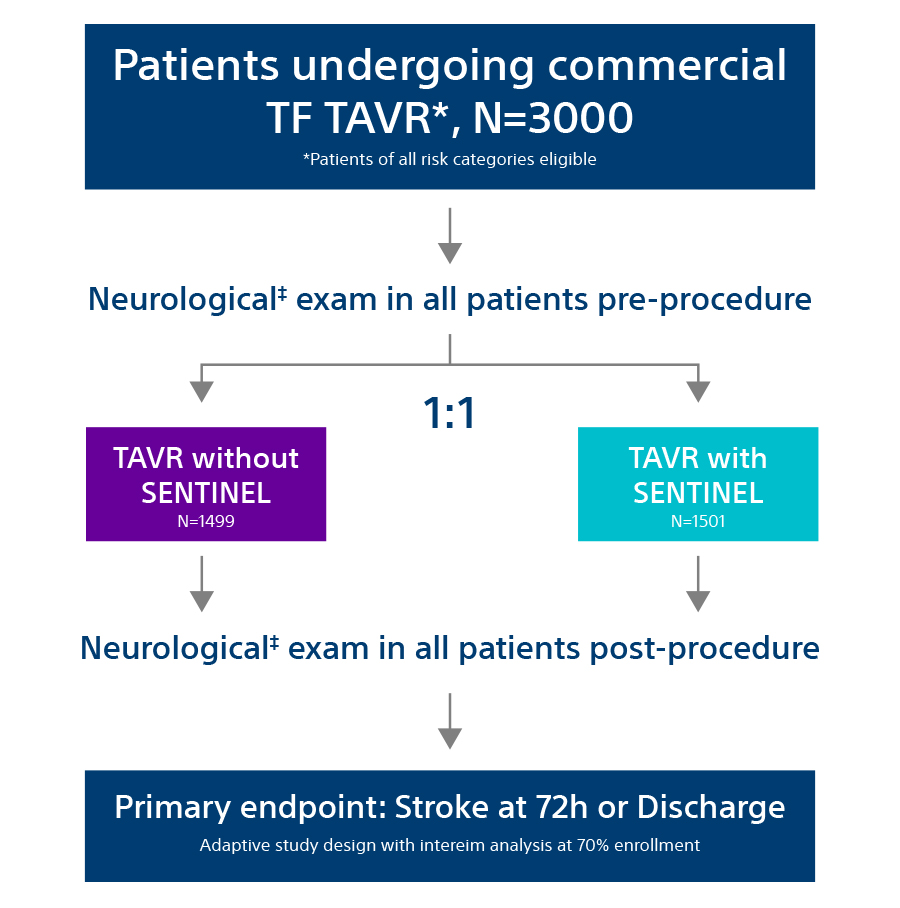
Study Design
The PROTECTED TAVR Study is the largest randomized TAVR trial to date with 3,000 patients enrolled at more than 50 global sites who were randomized 1:1 – patients protected with SENTINEL vs. no use of SENTINEL during TAVR. All risk categories were eligible for inclusion, including low risk patients and all commercially available valves were allowed as part of the trial.
Primary Endpoint: All Stroke (hemorrhagic, ischemic, or undetermined status; disabling or non-disabling) through 72-hours post TAVR procedure or hospital discharge.
Transient ischemic attack (TIA) and delirium were also reported on as part of the secondary neurological endpoints.
All patients enrolled in the trial underwent neurological examination at baseline and post TAVR procedure (discharge or 72-hours, whichever came first). This assessment was performed by a neurology professional (board certified/board eligible neurologist, neurology fellow, neurology physician assistant, or neurology nurse practitioner).
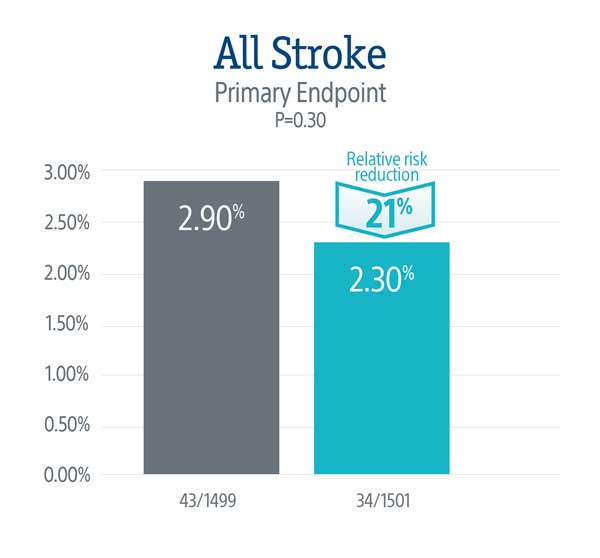
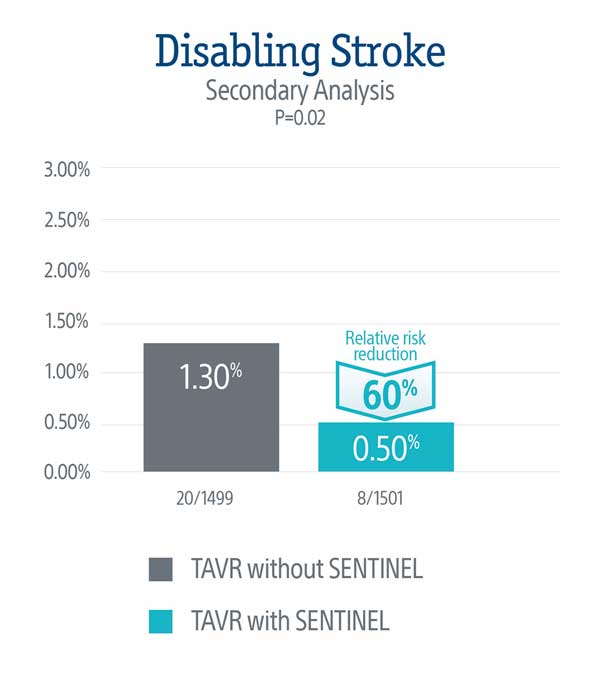
PROTECTED TAVR Results
The primary endpoint did not meet statistical significance, but the data demonstrated a numerical trend towards a lower rate of stroke in patients treated with the SENTINEL device, representing a 21% relative risk reduction in all stroke through 72-hours or time of hospital discharge.
A secondary analysis demonstrated a statistically significant 60% relative risk reduction in disabling stroke through 72-hours or time of hospital discharge in patients treated with the SENTINEL device.
Strong safety profile
The SENTINEL CPS demonstrated excellent safety profile with high rates of device delivery/retrieval (94.4%) and very low rates of vascular complications (0.1%).1
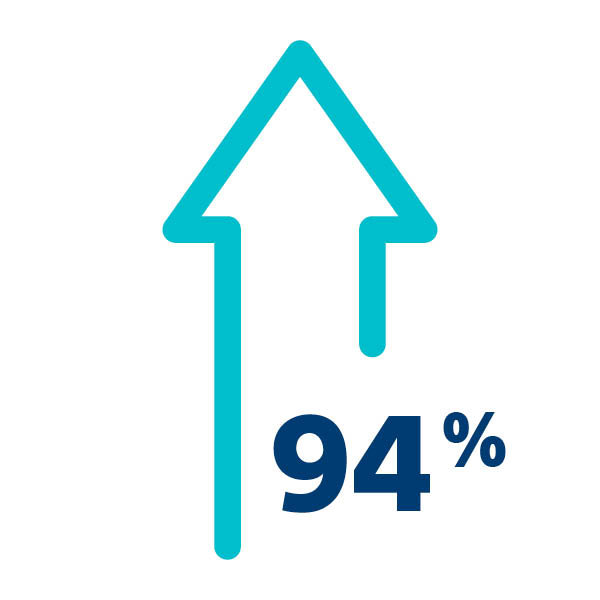
Safe & Effective Device Delivery

Low Access Site Complications
Stroke persists as an indiscriminate risk
It is impossible to predict which TAVR procedures will dislodge embolic debris, and when it will cause disabling stroke. 4 minutes per procedure are enough to achieve safe & effective device delivery.
Every patient is a candidate for protection from disabling stroke
The effect of SENTINEL on disabling stroke was consistent across patients' subgroups.
The PROTECTED TAVR Webinar
60 minutes interactive session moderated by Prof. Thomas Cuisset with TAVI experts Prof. Mohamed Abdel-Wahab, Prof. Lars Søndergaard and Dr. Joanna Wykrzykowska to learn more about :
- Understanding & interpreting PTAVR in a practical manner to reflect clinical practice.
- Addressing the role of CEP in Lifetime Patient Management for TAVI and focus on the periprocedural benefits of SENTINEL™.
IDE Trial
SENTINEL CPS is safe and fast to deploy
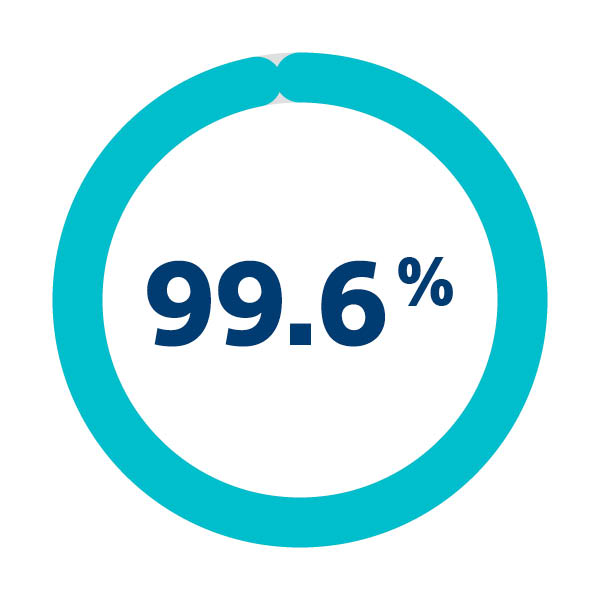
Safe and successful delivery and retrieval1
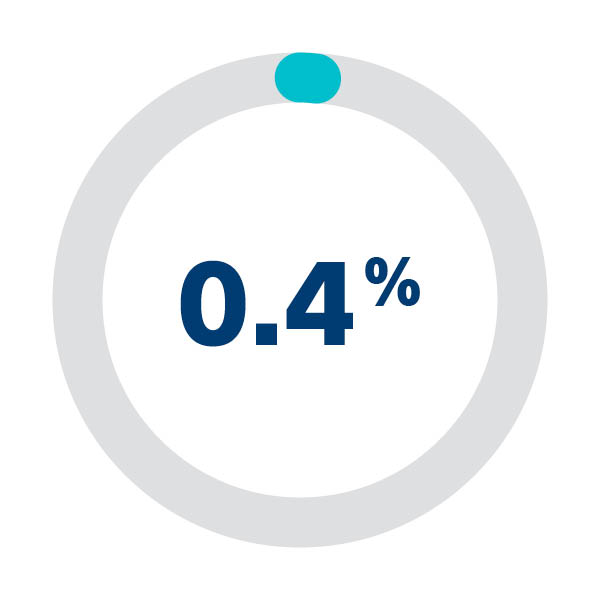
Access site-related vascular complication rate1
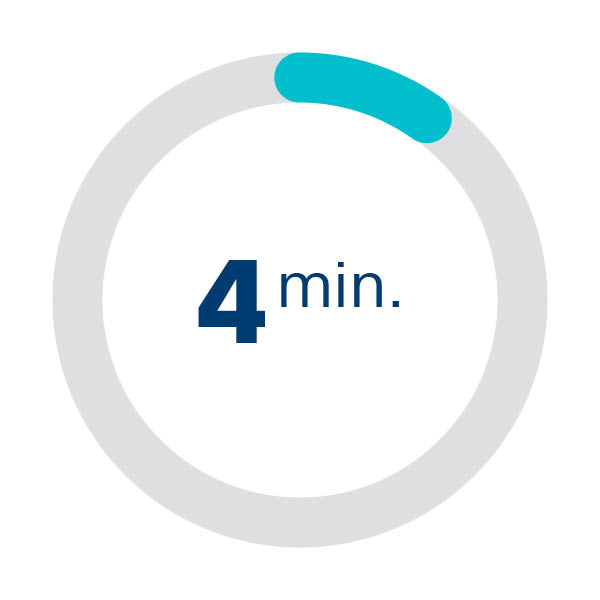
Median deployment time1
1. SENTINEL US IDE trial data presented at the SENTINEL CPS FDA Adv isory Panel, February 23, 2017
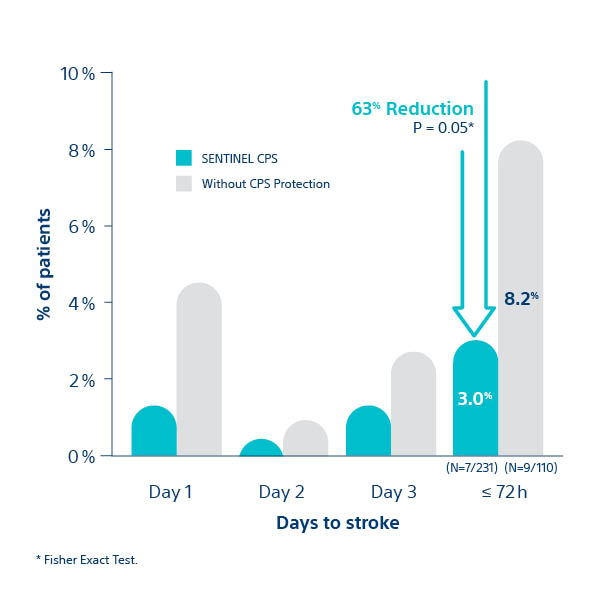
SENTINEL IDE Trial - Peri- Procedural Stroke Reduction
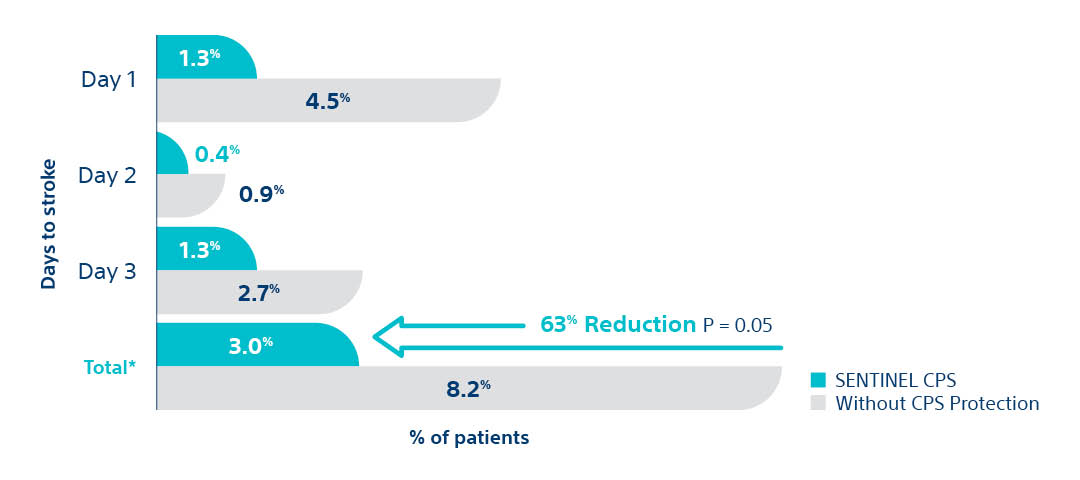
- Statistically significant 63% peri-procedural (< 72 hours) stroke reduction with SENTINEL™ CPS.
- 95% of SENTINEL CPS patients were evaluated by neurologists
- Clinical Events Committee included 2 stroke neurologists
SENTINEL IDE Trial. Data presented at SENTINEL FDA Advisory Panel, February 23, 2017
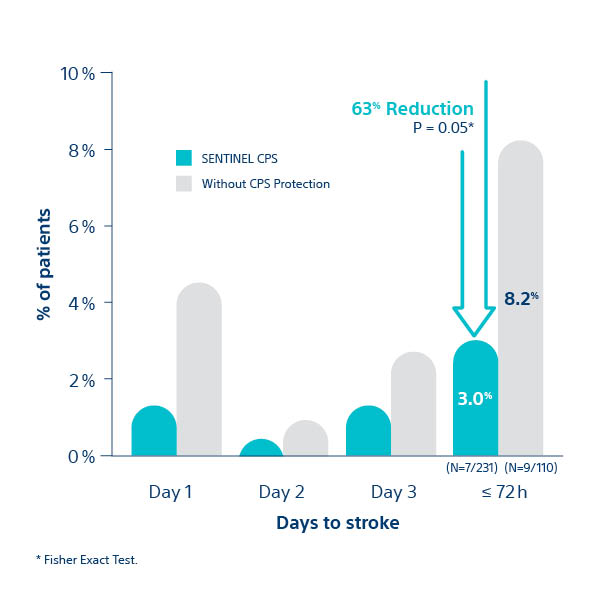
SENTINEL IDE Trial1
All Stroke at ≤ 72 hour post-TAVI
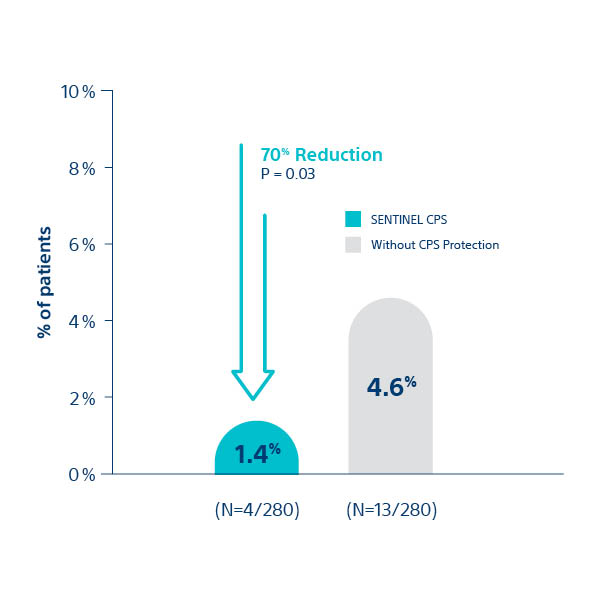
SENTINEL ULM STUDY2
All Stroke at 7 days post-TAVI
Erasmus and University Medical Centers3
All Stroke at ≤ 72 hour post-TAVI
Cedars Sinai Medical Center4
All Stroke at 7 days post-TAVI
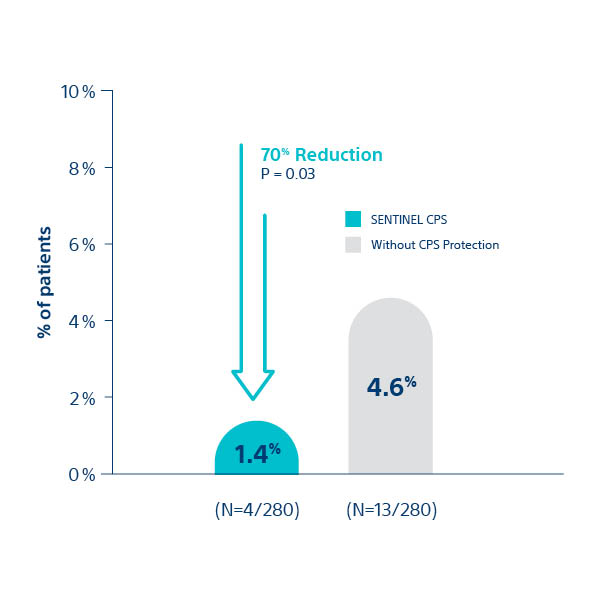
Findings from the SENTINEL IDE Trial Together with Real World Outcomes from Ulm, Erasmus and
Cedars Sinai Medical Centers Demonstrate Consistent Reductions in Stroke Among Nearly 2,400 Patients
1. Kapadia S, et al. J Am Coll Cardiol 2017;69:367–77; 2Seeger J et al. 2017. JACC Cardiovasc Interv. 10(22)2297-2303; 3van Mieghem N. presented at TVT 2018; 4Chakarvarty T. presented at TVT 2018
Debris found in majority of patients
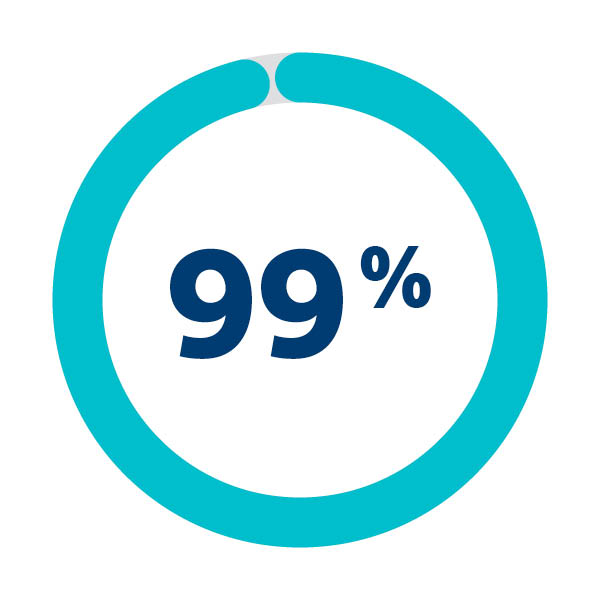
of patients in SENTINEL IDE trial were embolic debris were captured & removed1
1. Schmidt T et al. 2018. JACC Cardiov asc Intv . 11(13):1262-1273,
















