Three coils, Any case.
Just three coils in the EMBOLD™ Coil System support virtually any case, providing a complete portfolio that maximise economic value. Together, the EMBOLD™ Fibered Coil, the EMBOLD™ Packing Coil, and the EMBOLD™ Soft Coil reduce the number of coils on the shelf by half, simplify workflow, and streamline inventory.
- More efficient performance with multi-catheter compatibility, exceptional deliverability, and reduced inventory.
- Fewer coils to occlude means fewer coils per case. Plus, a comprehensive size matrix for high efficiency.
- Faster occlusion time for more optimised procedures.
Experience effortless efficiency
Create occlusions quickly with the Embold Coil System, using fewer coils overall to maximise procedural efficiency.1
Maximise occlusion power
The best-in-class thrombogenicity of the Embold Coil System requires less time and fewer coils to achieve accurate, consistent occlusion that maximises procedural efficiency. Offering the greatest metal volume of any .021” ID microcatheter compatible coils, the Embold Coil System consists of the most thrombogenic coils in their class.1 See how the Embold Coil System compares to other coil systems in a pre-clinical porcine model evaluating coils needed to achieve occlusion.2
5 vs 3 Coils
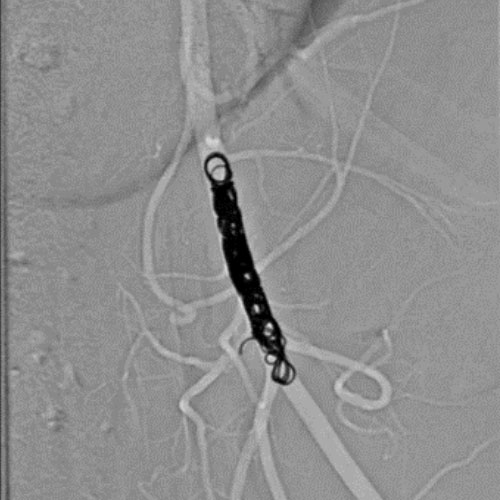 Ruby Standard Coil shown in left internal femoral artery
Ruby Standard Coil shown in left internal femoral artery
Left Internal Femoral
Artery
5 Ruby Standard 6x20
 Embold Fibered Coil shown in right internal femoral artery
Embold Fibered Coil shown in right internal femoral artery
Right Internal Femoral
Artery
3 Embold Fibered Coil 6x20
5 vs 3 Coils
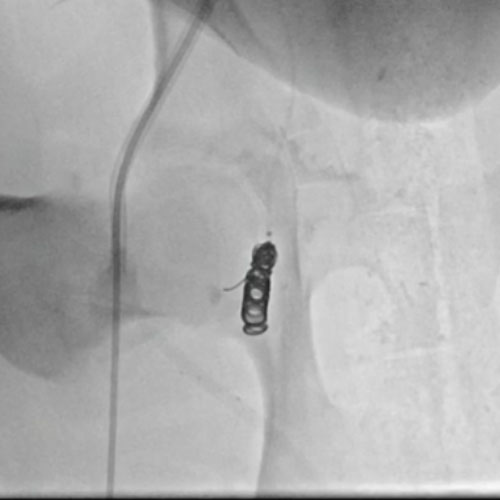
 Ruby Standard Coil shown in right internal iliac artery
Ruby Standard Coil shown in right internal iliac artery
Right Internal Iliac
Artery
5 Ruby Standard 6x20
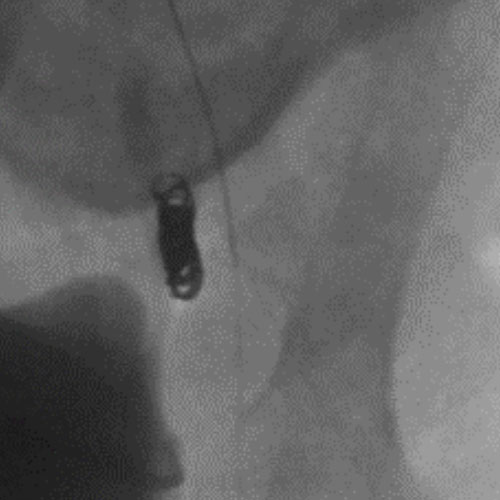 Embold Fibered Coil shown in left internal iliac artery
Embold Fibered Coil shown in left internal iliac artery
Left Internal Iliac
Artery
3 Embold Fibered Coil 6x20
3 vs 2 Coils
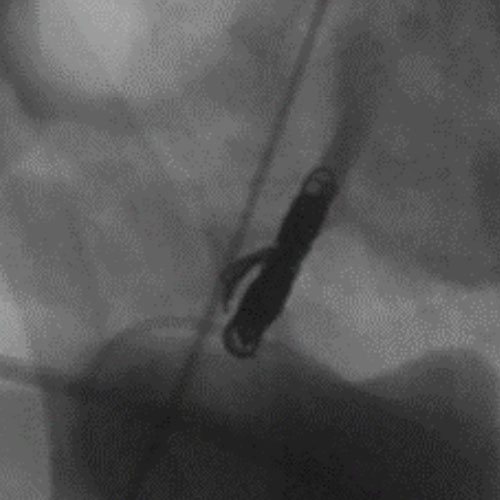
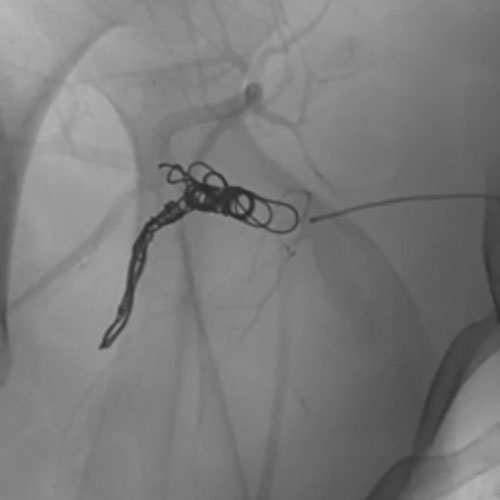 Ruby Standard Coil shown in right subclavian medial
Ruby Standard Coil shown in right subclavian medial
Right Subclavian
Medial
3 Ruby Standard 6x20
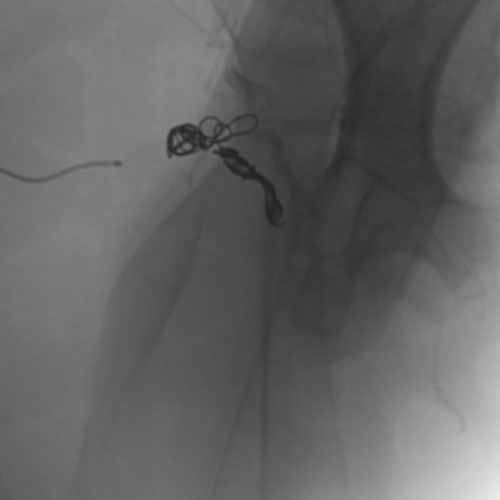 Embold Fibered Coil shown in left subclavian medial
Embold Fibered Coil shown in left subclavian medial
Left Subclavian
Medial
2 Embold Fibered Coil 6x20
Fewer coils to occlude
The Embold Coil System required about half the number of coils to occlude, likely due to its thrombogenicity and comprehensive size matrix with long lengths.
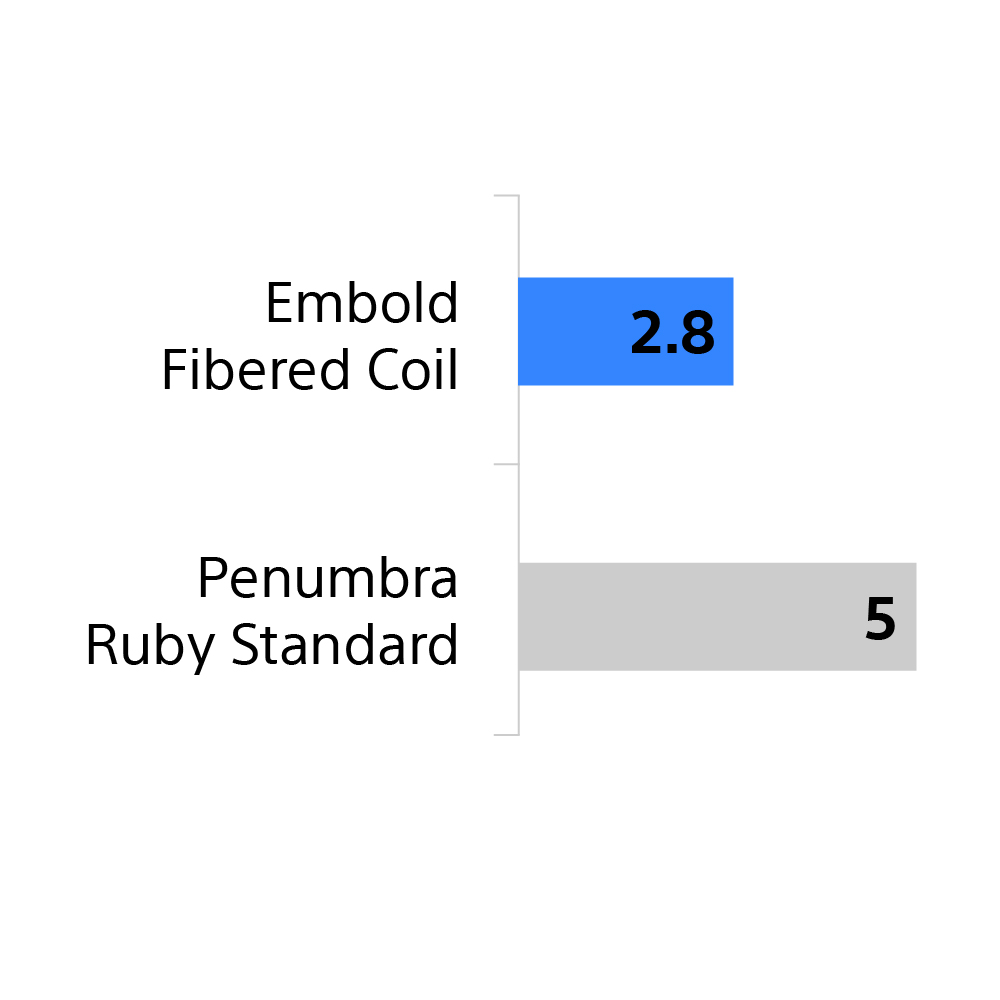
44% fewer
Embold Fibered Coils than Penumbra Ruby Standard
57% fewer
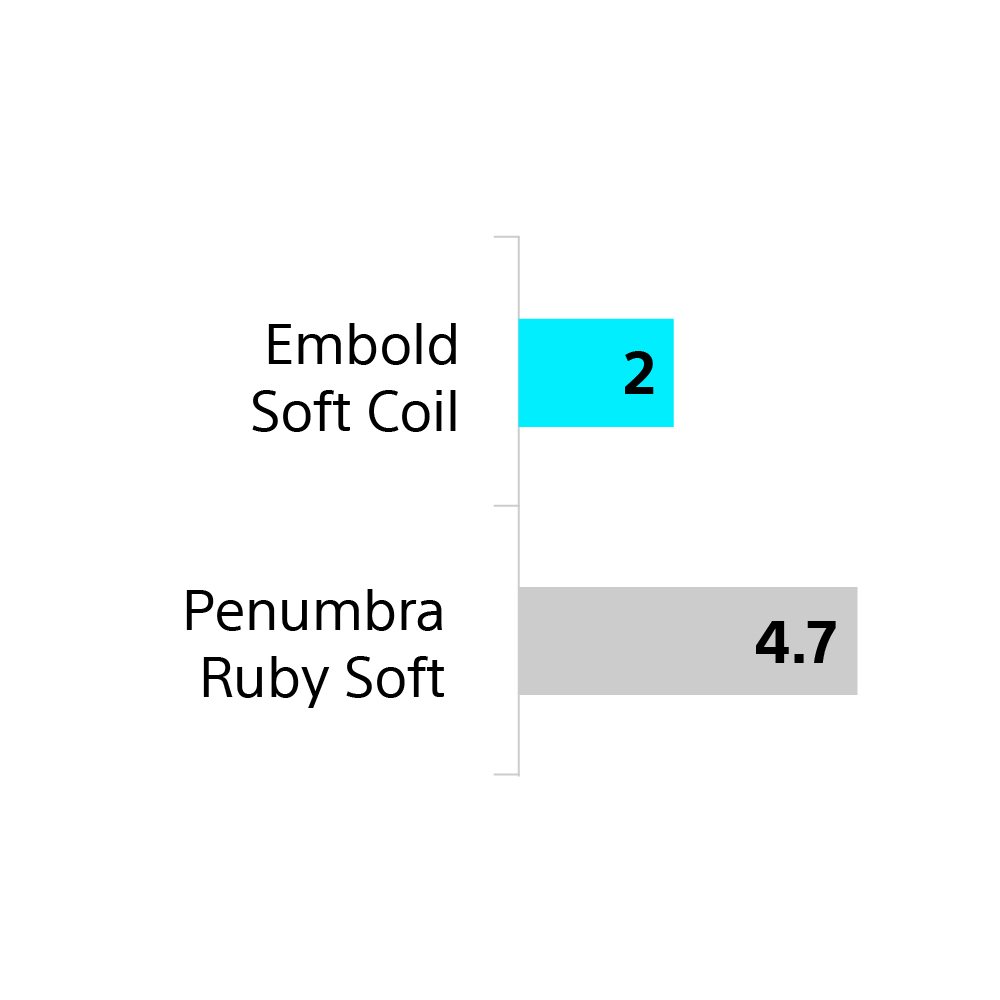
Embold Soft Coils than Penumbra Ruby Soft
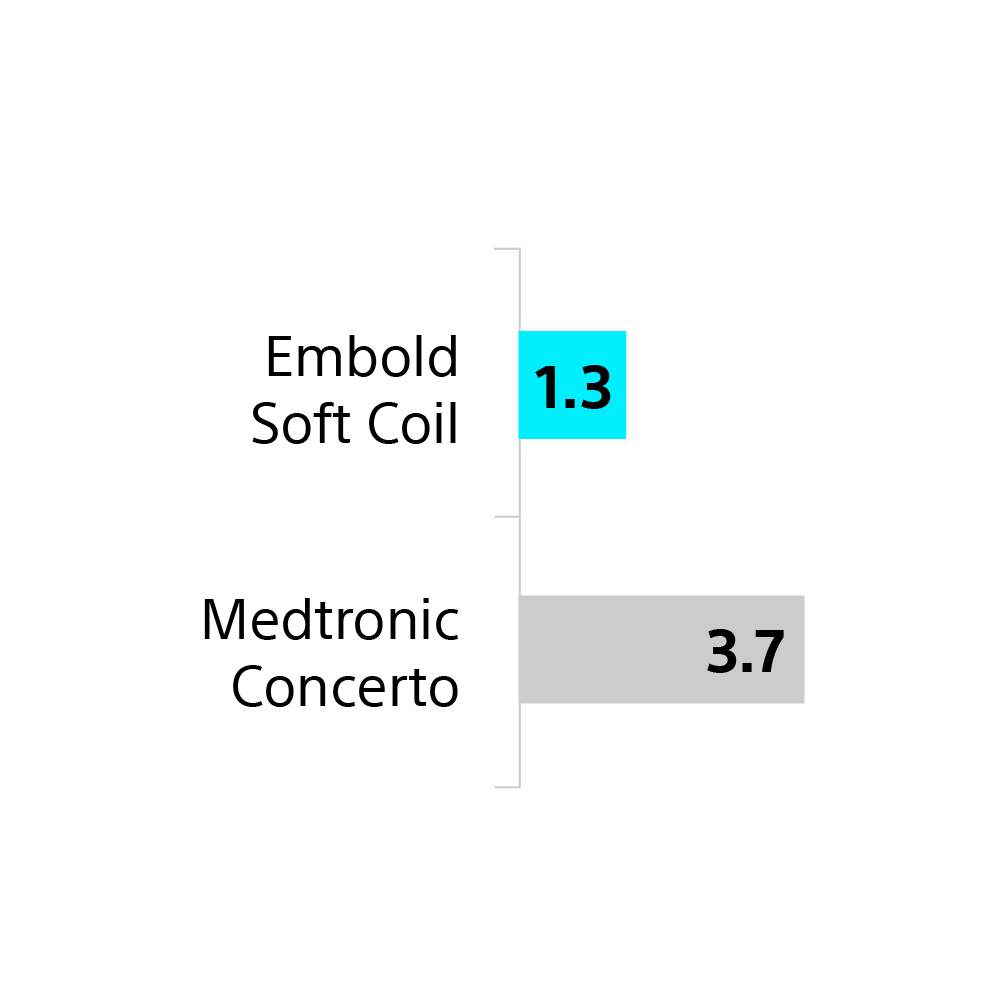
65% fewer
Embold Soft Coils than Medtronic Concerto
Faster occlusion time vs. competitors
Embold Fibered Coil Time to Occlusion2

Embold Packing Coil Time to Occlusion3
Embold Soft Coil Time to Occlusion4

Proven efficiency
- All three coils in the Embold Coil System accommodate .021” to .027” ID microcatheters, which provides procedural flexibility, simplified workflow, and streamlined inventory.
- Embold’s handle-free detachment eliminates the need for an ancillary device, saving time and cost during the procedure.
- With a Nitinol delivery system that resists kinking, the Embold Coil System is designed for exceptional deliverability and has a lower risk of premature detachment.
1. The testing was performed by or on behalf of BSC. Data on file.
2. Evaluation conducted in a pre-clinical porcine model. Coils were deployed every 3 minutes until complete occlusion was achieved. Complete occlusion was defined as TIMI Grade 0 (no antegrade flow beyond the point of occlusion) and assessed by the physician evaluator. Contralateral vessel pairs of comparable diameters were used for each assessment. For EMBOLD Soft comparisons, 3 replicates were conducted. For EMBOLD Fibered, 4 replicates were conducted. For Embold Soft comparisons, the longest coil length commercially available for each coil type was used: Concerto 4mm x 10cm/Embold Soft 4mm x 30cm and Ruby Soft 6mm x 30cm/Embold Soft 6mm x 60cm. For Embold Fibered, comparable coil lengths were evaluated to assess fiber vs. metal impact: Ruby Standard 6mm x 20cm/Embold Fibered 6mm x 20cm. med six times. Testing done at an independent facility. Bench Test study results may not necessarily be indicative of clinical performance.
3. In vitro bench model: testing results from bovine blood flow loop. Occlusion time is an average of a single 6 x 20 coil from each manufacturer performed six times. Testing done at an independent facility. Bench test or pre-clinical study results may not be necessarily indicative of clinical performance.
4. In vitro bench model: testing results from bovine blood flow loop. Occlusion time is an average of a single 30 cm coil from each manufacturer performed six times. Testing done at an independent facility. Bench Test study results may not necessarily be indicative of clinical performance.
5.In vitro bench model: testing results from bovine blood flow loop. Occlusion time is an average of a single 3 x 15 coil from each manufacturer, except for Concerto with two 3 x 8 coils, performed six times. Testing done at an independent facility. Bench Test study results may not necessarily be indicative of clinical performance.
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.