Medical Specialties > Interventional Radiology > Ablation Solutions > Cryoablation Indications > Renal Cell Carcinoma
Renal Cell Carcinoma
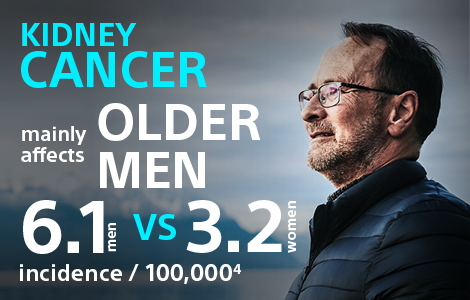
Kidney cancer is the 7th most common cancer in men, representing 5% of all malignancies, and the 10th most common cancer in women (3% malignancies).1 More than 90% of kidney cancer diagnoses are renal cell carcinoma (RCC), with the main histological subtype being clear-cell – the most aggressive form.2, 3
The incidence rate of RCC is growing (about 2.4% per year), partly due to an increase in the incidental detection of small lesions (<4 cm)4
Depending on the stage of severity, kidney tumours are classified into1:
- stage I: the tumor is limited to the kidney with a maximum diameter of 7 cm, and in particular:
- StageT1a (≤4 cm);
- Stage T1b (4-7 cm);
- stage III: the tumour is no longer confined to the kidney, but there are no distant metastases;
- stage IV: cancer has metastasised to other parts of the body.

Subtypes
Tumour Subtype |
Incidence |
Description |
Use of CA |
||
Clear-cell RCC |
75% A |
|
|||
Non clear-cell RCC |
Papillary RCC |
10% A |
|||
Chromophobe RCC |
5% A |
||||
Treatment options
Local treatments
|
|
|
|
|
Surgery (partial nephrectomy) |
Active Surveillance |
Radiation Therapy |
RF Ablation |
Systemic treatments
|
|
|
Targeted Drug Therapy |
Immunotherapy |
Chemotherapy |
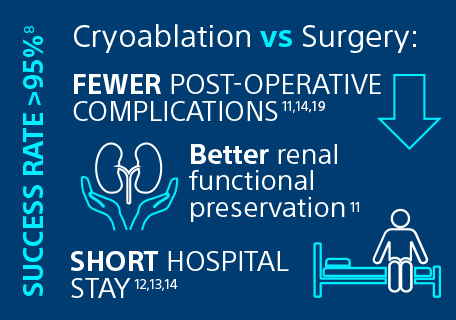
Recently, cryoablation has been increasingly recognised by urologists, oncologists, and interventional radiologists as a preferred treatment for RCC. Indeed, percutaneous cryoablation is now endorsed by the European Association of Urology (EAU) as a highly effective technique in terms of success rates (> 95%)8, particularly in patients with stage 1a or 1b RCC8, 9, 10.
Clinical Results – Why Cryoablation?
Discover the benefits of cryoablation VS surgery for renal cell carcinoma (RCC)
«Cryoablation is a safe and precise ablation procedure. Cryoablation provides urologists and IRs with more therapeutic options that the dedicated teams can offer to the patient with results comparable to surgery.»
Prof. Rosario Francesco Grasso, Policlinico Universitario Campus Bio-Medico, Italy
Cryoablation has been shown to be an effective alternative to surgery for the treatment of stage I RCC.8, 9 It not only provides better overall survival rates than partial nephrectomy (72% vs 49% at 10-year)9, but also offers lower post-operative complications rate (15% vs 31%) 11, 14, 19, superior renal function preservation11 and a shorter hospital stay (1 day vs 4/5 days)12, 13, 14.
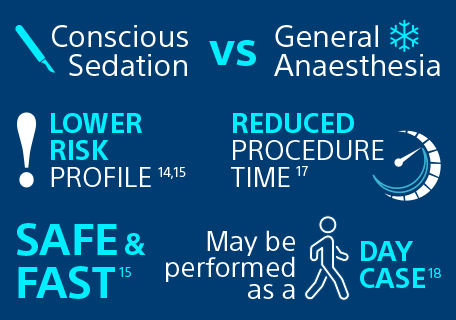
Discover the benefits of treating RCC under local anaesthesia conscious sedation (LACS)
“Percutaneous cryoablation under local anaesthesia conscious sedation is an efficacious and safe procedure for managing small renal masses with a low complication and treatment failure rate, similar to that seen in series using general anaesthesia.”
Patel SR, Francois S et al. 202015
A key benefit of cryoablation for the treatment of renal cell carcinoma is that it can be performed under local anaesthesia conscious sedation, providing the same outcomes as when performed under general anaesthesia in terms of safety (low treatment failure rate) and efficacy14, but also reducing the costs16, the procedure time (102 mins) and length of hospital stay (average 1.08 days)17. In many centres, it is performed as a day case)18.
Our Solutions
Cryoablation is a minimally invasive treatment that uses extreme cold to freeze and destroy diseased tissue, including cancer cells. We offer a broad portfolio of Systems and Needles, that, thanks to the latest technology, provide an efficient treatment against a range of malignant and benign tumours.
Learn more about our solutions
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, Warnings and instructions for use can be found in the product labelling supplied with each device. Information for the use only in countries with applicable health authority product registrations. Material not intended for use in France.