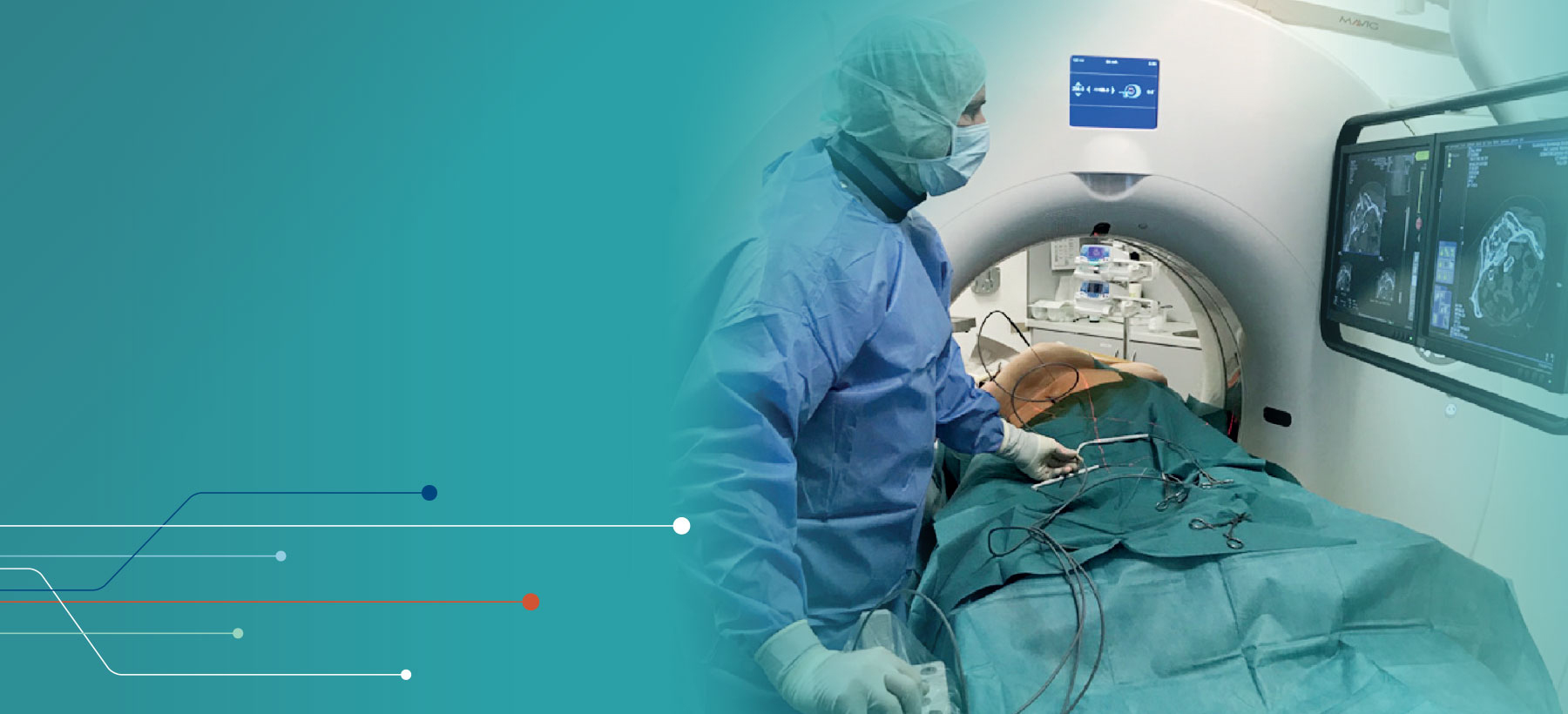
Medical Specialties > Interventional Radiology > Ablation Solutions
Ablation Solutions
Boston Scientific is committed to supporting Interventional Radiologists and their patients. With significant investments in new technologies, product pipeline growth and focus on expanding indications, we can offer a best in class product portfolio.
From market-leading cryoablation systems offering flexibility and control to our radiofrequency product for soft tissue ablation, we offer options for any case.
- RFA – a long-lived technique that treats solid cancer by creating a localised region of heat through high-frequency electrical currents. RF 3000™ Radiofrequency Ablation System is simplicity and predictability in soft tissue ablation.
- Cryoablation – an innovative technology that alternates freezing and thawing to destroy localised cancer cells. The ICEFX™ and VISUAL ICE™ are intended for the cryoablative destruction of tissue during minimally invasive percutaneous procedures.
What is Cryoablation?
What are the benefits for clinicians?
Cryoablation offers clinicians a number of unique advantages:
- Treatment zone visibility and control
- Ability to treat multiple tumours in one session
- Ability to use ice in proximity to critical structures / vasculature
- Ability to use multiple probes to “sculpt” the shape of the iceball and ablate larger tumours
- Repeatability of the treatment
- Repeatability
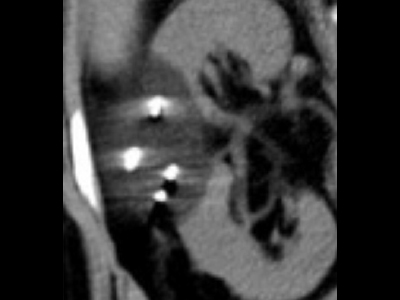
What are the benefits for patients?
How is the iceball created?
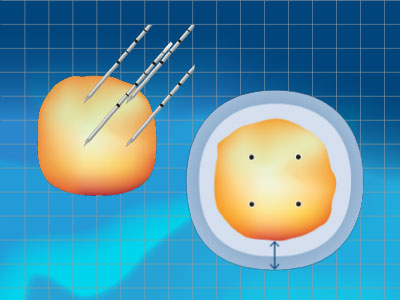
Intraoperative imaging is important to monitor iceball formation throughout the procedure and is key to a successful cryoablation.
- To optimise tumour coverage and provide appropriate margins, the use of multiple needles is recommended. Multiple needles placed in an adjacent configuration will typically create a large, coalesced iceball.
- To optimise appropriate margins, needles should be placed to create lethal ice beyond the perimeter of the target tissue (5-10mm, depending on tissue type).
Clinical Results

92% (59/64) of patients achieved pain palliation
-2.61 points in pain score in 8 weeks!
Our Indications
 |
 |
 |
 |
 |
RENAL CELL |
SOFT TISSUE TUMOURS |
BONE |
LUNG |
PROSTATE |
Our Solutions
The ICEfx™ Cryoablation System the next generation of ablation systems
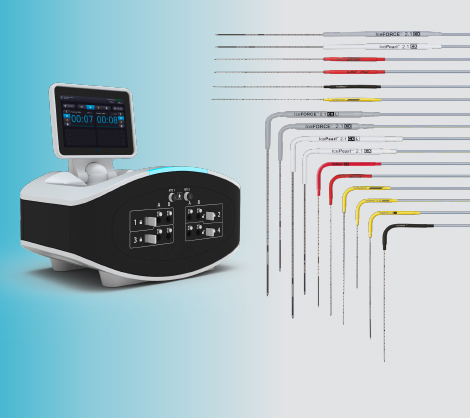
Radiofrequency Ablation
Designed for use with the RF3000™ Radiofrequency Generator
- Umbrella-shaped array
- Wide portfolio matrix of array diameters from 2cm to 5cm
- Soloist Single Needle Electrode for small 1.5cm x 1cm ablation zones
- The only RFA system that utilizes impedance to accurately assess the procedural endpoint
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, Warnings and instructions for use can be found in the product labelling supplied with each device. Information for the use only in countries with applicable health authority product registrations. Material not intended for use in France.