Patient case: Saul

Discover a patient’s gastrointestinal stromal tumour journey developed in collaboration with clinician experts.
Start of symptoms
Saul Rosenberg, a 60-year-old male, sought medical attention in the Emergency Department after experiencing a fainting episode accompanied by abdominal discomfort. Hematological evaluation identified anemia, leading to the administration of a blood transfusion. Saul was then referred for an esophagogastroduodenoscopy (EGD).
A lesion, measuring around 6 cm in width and displaying ulceration, was detected on the gastric wall of the stomach during the EGD examination. Furthermore, blood clots were observed within the stomach. The visual characteristics of the lesion raise suspicion of a gastrointestinal stromal tumor (GIST).
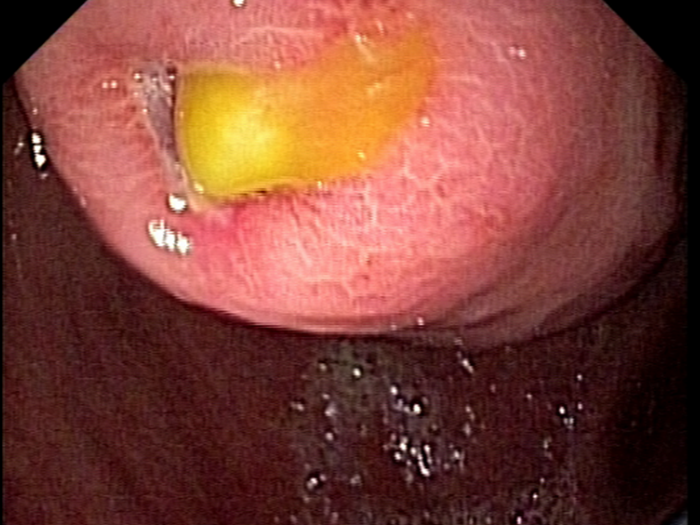
As a result, Saul is referred for an endoscopic ultrasound (EUS)-guided fine-needle biopsy (FNB) for further diagnostic evaluation.
Saul’s diagnosis

1. EUS-guided FNB
Saul was consented to move forward with an EUS-guided FNB, that was performed with Acquire™ S. This allowed his gastroenterologist to further assess the lesion. A 51.9 x 47.9 mm hypoechoic, inhomogeneous lesion, which appears hypervascularised on Hi Flow, is observed. Unfortunately, an advanced, unresectable GIST is still suspected.
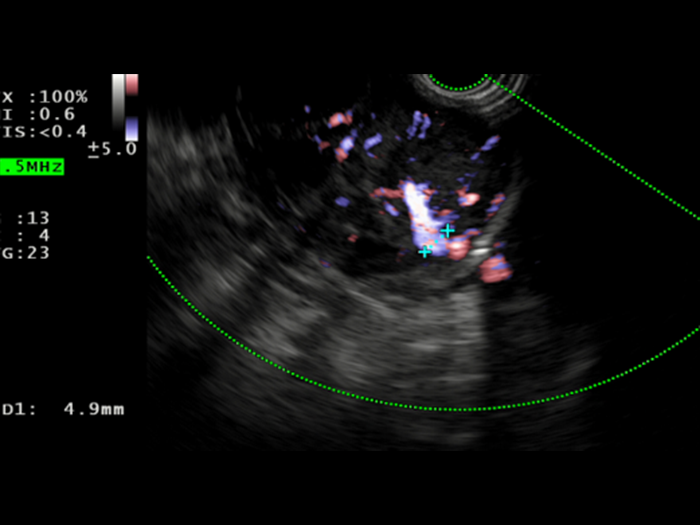
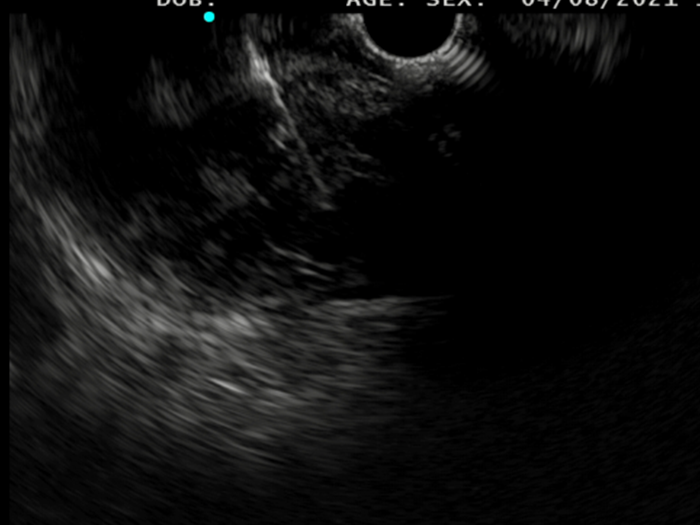

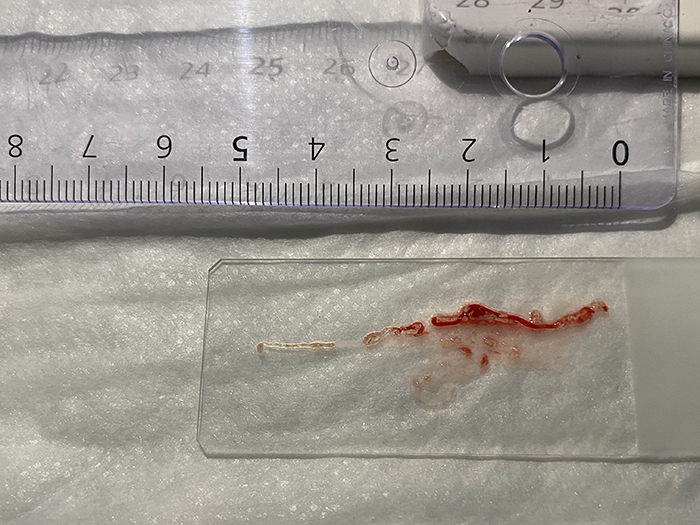
2. Macroscopic on-site evaluation
The ESGE guidelines suggest two to three passes with an FNB needle. This number of passes in addition to the fanning technique will result in multiple parts of the lesion being assessed, hence increasing diagnostic accuracy. After each pass, the medical team in charge of Saul performed a macroscopic on-site evaluation (MOSE). This allowed for the quick and direct examination of the sample to confirm that enough tissue was collected, without requiring a pathologist on site.
After only two passes white tissue and only small amounts of blood were observed during MOSE, meaning the EUS-guided FNB was successful in acquiring enough tissue for histological analysis. The sample was then sent for further analysis.
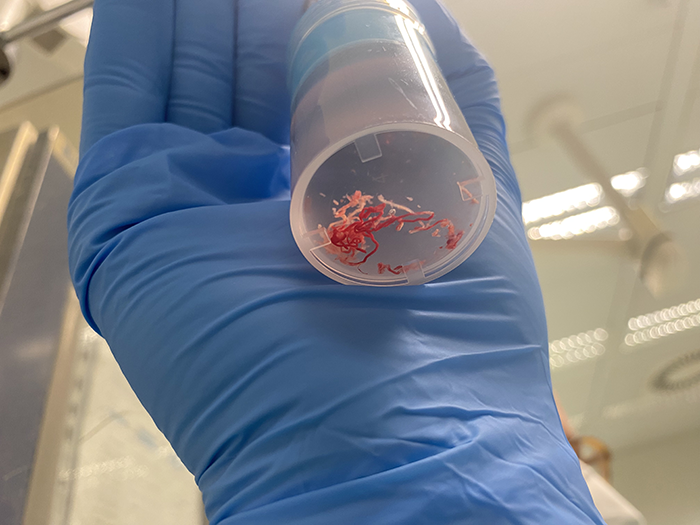
3. Next Generation Sequencing
Following discussion with the multidisciplinary team (MDT), including an oncologist, Saul’s medical team agreed to further investigate their initial diagnosis of an advanced, unresectable GIST by requesting Next Generation Sequencing (NGS) analysis of the sample. NGS approach is feasible for routine mutational analysis of GIST 1, so to allow early identification of the lesion’s specific molecular profile, which could influence the chosen therapy.
4. Targeted therapy of GIST
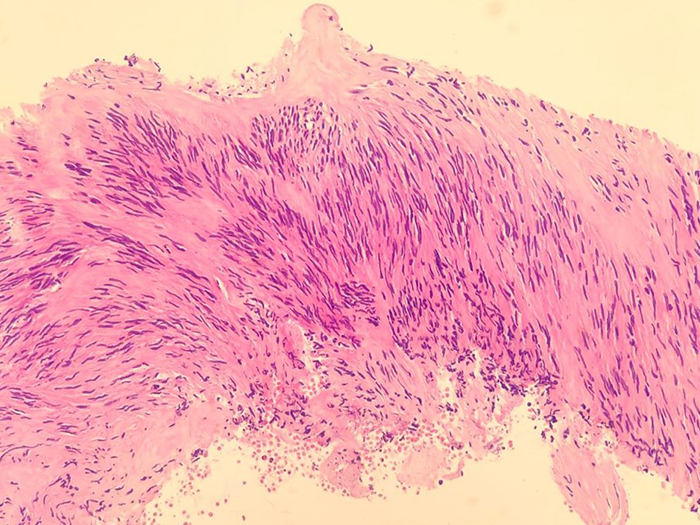
The pathologist reviewed the tissue sample and identified cells with spindled morphology, confirming the diagnosis of a GIST. NGS results revealed no KIT mutation on exon 11, which is the cause for approximately 80% of all GISTs.2
Instead, NGS analysis showed a mutation on PDGFr-A. This GIST’s genetic profile means Saul would not respond to the classic first line treatment.
Tailoring therapy to Saul's needs

The oncologist proceeded with a targeted therapy suitable for Saul’s tumour’s mutation profile. This treatment was given for 8 months until the disease was stable.
















