EXALT™ Model D Single-Use Duodenoscope Generation 4
Raise the standard of care with the fourth-generation EXALT Model D, the only single-use duodenoscope with seven peer-reviewed, published studies evaluating feasibility, safety and performance.
This new generation is designed to deliver both the feel and performance that physicians have come to expect from a reusable scope. With this groundbreaking single-use platform, EXALT Model D eliminates patient-to-patient infection risk due to cross-contamination from ineffective reprocessing. All while reducing associated operational costs for endoscopic retrograde cholangiopancreatography (ERCP) procedures. Model D platform enhancements are the results of a commitment to quality and a continuous improvement approach.

Force
reduction

Enhanced
ergonomics
Reduce
infection risk
No reprocessing required
Installation & support

Peer-reviewed trialsReview the data per peer-reviewed, published data and hear what leading physicians have to say about the results. *Grades as defined by the American Society for Gastrointestinal Endoscopy |

CASE STUDIES
Meeting the needs of
ASGE complexity grades
See how the EXALT Model D Single-Use
Duodenoscope delivers robust stability,
maneuverability and control in ERCP
procedures.

ECONOMIC IMPACT
An economically viable solution
Learn how EXALT Model D provides an economically viable alternative to reusable duodenoscopes.
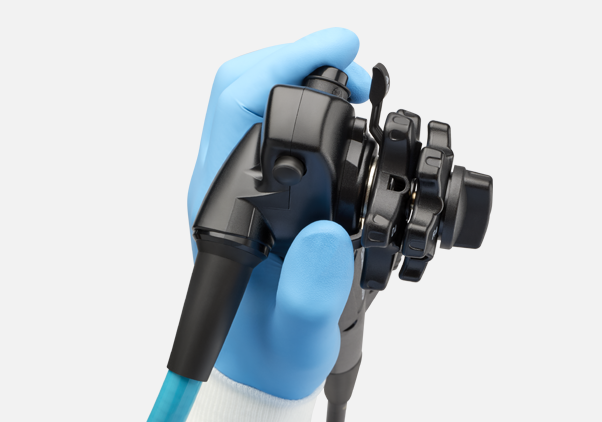
Built for performance
EXALT Model D offers the performance, control and maneuverability you need in a single-use platform.
Prevent infection
EXALT Model D eliminates patient-to-patient infection risk due to ineffective duodenoscope reprocessing.
EXALT™ Model D
Single-Use Duodenoscope
delivers robust stability, maneuverability
and control in ERCP procedures.
![]()
Stay up to date
Sign up to receive periodic emails about EXALT Model D case studies, clinical data, reimbursement and more.
![]()
Connect with a rep
Request a rep to learn how EXALT Model D may help you address infection risks and improve patients’ lives.


















