Capacity + Chemistry + Efficiency

Unparalleled Capacity
EnduraLife Battery Technology has the largest usable battery capacity in the industry.
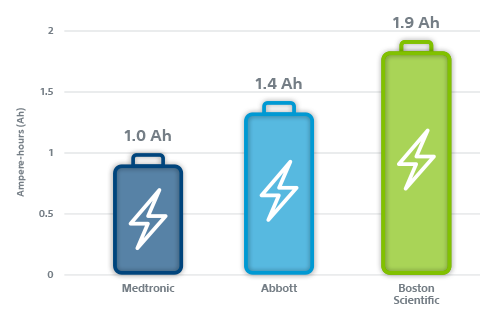 Boston Scientific EL ICDs and X4 CRT-Ds with ENDURALIFE Battery Technology provide nearly 2x more usable capacity than all Medtronic ICDs and CRT-Ds.1
Boston Scientific EL ICDs and X4 CRT-Ds with ENDURALIFE Battery Technology provide nearly 2x more usable capacity than all Medtronic ICDs and CRT-Ds.1
Smarter Chemistry
Li/MnO2 chemistry maintains stable operating voltage and internal resistance for effective battery utilization.
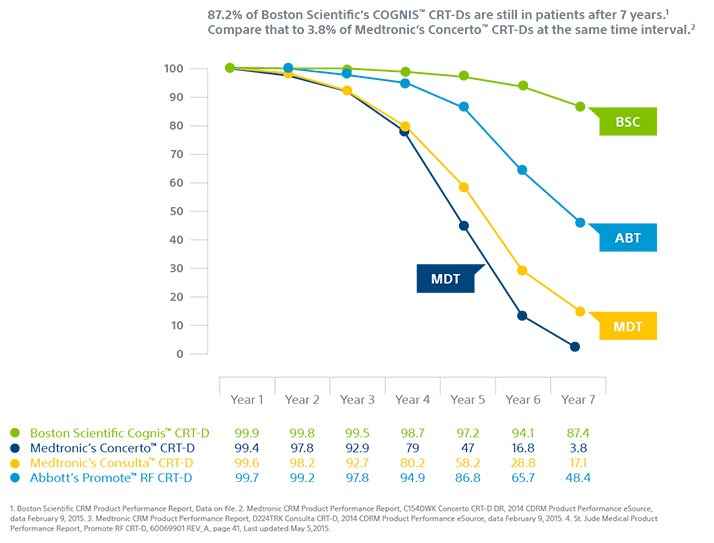
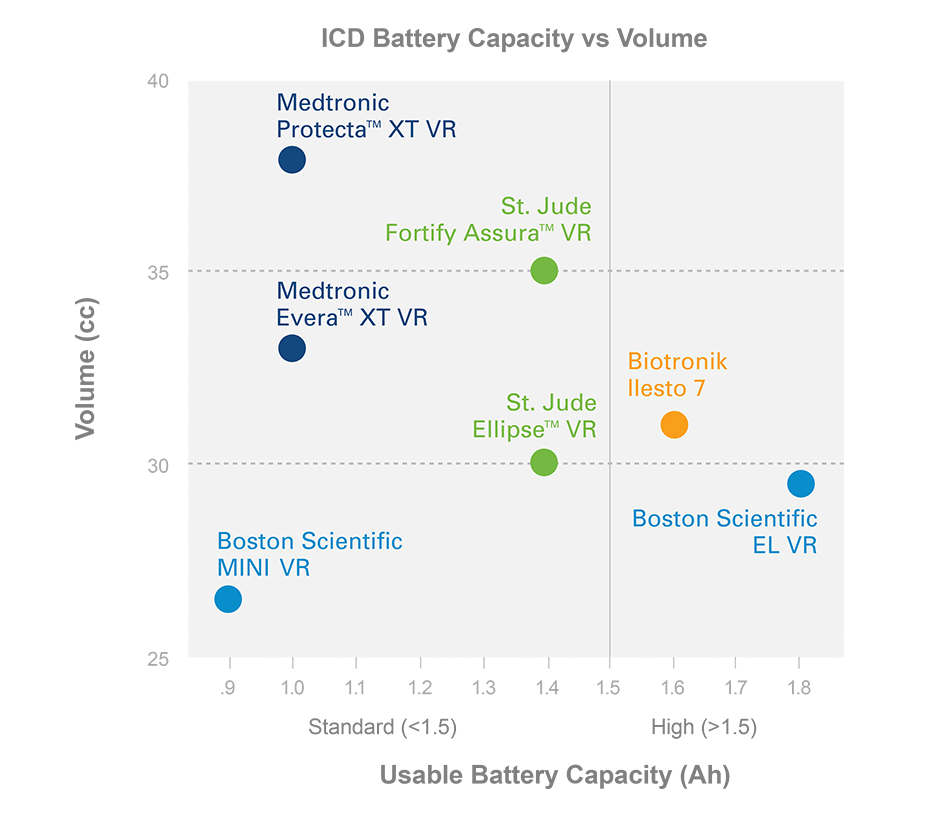
Greater Efficiency
Advanced manufacturing capabilities enable a device that is up to 11% smaller, 24% thinner and has nearly twice the usable battery capacity of Medtronic devices.¹,²
We're here to help
References:
- Medtronic ICDs and CRT-Ds have 1.0 Ah. Amplia MRI CRT-D Surescan DTMB2D4 UK 2016 Manual pg 30. Viva XT CRT-D OUS Manual DTBA2D1 2013 page 28. Evera XT DR ICD OUS Manual DDBB2D4 2014 page 28. Cobalt XT VR on manual M975368A001 REV. C 2020 pag 26. ABBOTT High Voltage Devices Based on battery discharge testing performed by BSC, data on file. DYNAGEN™ EL ICD, DYNAGEN™ MINI ICD, INOGEN™ EL ICD, INOGEN™ MINI ICD, ORIGEN™ EL ICD, ORIGEN™ MINI ICD Physician's Technical Manual. Part Number: 359050-003 EN US 2014-01. DYNAGEN™ CRT-D, DYNAGEN™ X4 CRT-D, INOGEN™ CRT-D, INOGEN™ X4 CRT-D, ORIGEN™ CRT-D, ORIGEN™ X4 CRT-D Physician's Technical Manual. Part Number: 259049-004 EN US 2014-04.RESONATE, MOMENTUM, CHARISMA, VIGILANT, PERCIVA VR Physician's Technical Manual Part Number: 360199-003 2019 pag 34
- PHYSICIAN’S TECHNICAL MANUAL DYNAGEN™ EL ICD, DYNAGEN™ MINI ICD, INOGEN™ EL ICD, INOGEN™ MINI ICD, ORIGEN™ EL ICD, ORIGEN™ MINI ICD 2014, page 27-29. PROTECTA™ XT VR D314VRM 2013, page 330. RESONATE, MOMENTUM, CHARISMA, VIGILANT, PERCIVA VR Physician's Technical Manual Part Number: 360199-003 2019 pag 32. EVERA™ XT VR DVBB1D4 2013, page 24. Cobalt XT VR on manual M975368A001 REV. C 2020 pag 24. AnalyST™, AnalyST Accel™, Current™, Current Accel™, Fortify™, Fortify™ ST, Promote™, Promote Accel™, Promote™ Q, Unify™ Devices User’s Manual 2013, page 29. St. Jude Medical™ High-Voltage Devices User’s Manual 2013 page 16. Avant™, Neutrino™ NxT, Gallant™, Entrant™ device user´s manual 2020 page 54.
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Information for use only in countries with applicable health authority registrations. Material not intended for use in France.
All cited trademarks are the property of their respective owners.
















