Outperforming the Competition
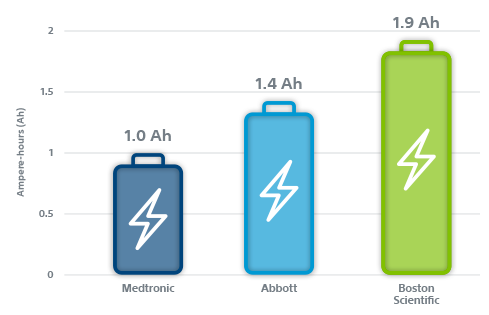 Boston Scientific EL ICDs and X4 CRT-Ds with ENDURALIFE Battery Technology provide nearly 2x more usable capacity than all Medtronic ICDs and CRT-Ds.1
Boston Scientific EL ICDs and X4 CRT-Ds with ENDURALIFE Battery Technology provide nearly 2x more usable capacity than all Medtronic ICDs and CRT-Ds.1

Clinically Meaningful Innovation
Backed by nine clinical trials³⁻¹¹ and over seven years of real-world data, EnduraLife Battery Technology consistently demonstrates industry-leading longevity performance. In addition to having the longest labeled projected longevity, these innovative ICD and CRT-D devices come equipped with 90% more usable battery capacity than Medtronic defibrillators.
A Historical Stand To Do Better

Boston Scientific made a historic choice in 2002 to start investing in the design of longer-lasting devices - a choice that benefits both patients and physicians. EnduraLife Battery Technology powers a dozen Boston Scientific device families with a dependable, durable energy source that continues to outlast the competition.
We're here to help
References:
- ESC Guidance for Diagnosis and Management of CVD during the COVID-19 Pandemic: https://www.escardio.org/Education/COVID-19-and-Cardiology.
- 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC, European Heart Journal (2021) 00, 1-128 doi:10.1093/eurheartj/ehab368.
- 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA), European Heart Journal (2021) 00, 1-94 doi:10.1093/eurheartj/ehab364.
- Haarbo J, Hjortshoj S, Johansen J, Jorgensen O, Nielsen J, Petersen H. Device Longevity in Cardiac Resynchronization Therapy Implantable Cardioverter Defibrillators Differs Between Manufacturers: Data from the Danish ICD Registry. Presented at HRS 2014. https://ondemand.hrsonline.org/common/presentation-detail.aspx/15/35/1241/9000 . Boston Scientific = 136 patients, Medtronic = 651 patients, St. Jude Medical = 1,587 patients, Bitronik = 369 patients. Time to exchange of the device because of battery depletion or device failure recorded in the Danish ICD Registry was the endpoint. The four-year survival rate for devices in the Danish Registry study was 81.1% for Medtronic and 95.7% for Boston Scientific (P<0.01).
- J. Williams, R. Stevenson. Contemporary cardiac resynchronization implantable cardioverter defibrillator battery longevity in a community hospital heart failure cohort. Presented at HFSA 2014. https://www.onlinejcf.com/article.S1071-9164(14)00389-3/fulltext . Boston Scientific = 53 patients, Medtronic = 28 patients, St. Jude Medical = 10 patients. Four-year survival rate calculated using device replacements for battery depletion as indicated by ERI.
- Ellis CR, Dickerman DI, Orton JM, Hassan S, Good EG, Okabe T, Andruilli JA, Quan KJ, Greenspon AJ. Ampere Hour as a Predictor of Cardiac Resynchronization Defibrillator Pulse Generator Battery Longevity: A Multicenter Study. PACE 2016 doi: 10.1111/pace.12831 first published online 11-MAR-2016. The five major institutions performing the study include, at Vanderbilt University, Henry Ford Hospital, University of Michigan, Thomas Jefferson University, Cooper Health System, North Ohio Heart Center. Boston Scientific = 322 patients, Medtronic = 794 patients, St. Jude Medical = 186 patients. Five-year survival rate calculated using device replacements for battery depletion as indicated by ERI.
- Landolina M, Curnis A, Morani G, Vado A, Ammendola E, D’onofrio A, Stabile G, Crosato M, Petracci B, Ceriotti C, Bontempi L, Morosato M, Ballari GP, Gasparini M. Longevity of implant Cardioverter-defibrillators for cardiac resynchronization therapy in current clinical practice: an analysis according to influencing factors, device generation, and manufacturer. Europace 2015;17:1251-58. doi:10.1093/eurospace/euv109. First published online: May 8, 2015. Medtronic = 532 patients, Boston Scientific = 291 patients, St. Jude Medical = 106 patients, Biotronik = 20 patients, Sorin = 69. Five-year survival rate of latest marketed devices (between 2006 to 2010) calculated using device replacements for battery depletion as indicated by ERI.
- Zanon F, Martignani C, Ammendola E, Menardi E, Narducci ML, De Filippo P, Santamaria M, Campana A, Stabile G, Potenza DR, Pastore G, Iori M, La Rosa C, and Biffi M. Device Longevity in a Contemporary Cohort of ICD/CRT-D Patients Undergoing Device Replacement. Doi:10.1111/jce.12990, First published online 20-APR-2016. Comparison of device longevity by Kaplan-Meier curves of CRT-D systems extracted between March 2013 and May 2015. Medtronic = 195 patients, Boston Scientific = 157 patients, St. Jude = 72, Biotronik = 9.
- Provided by Dr. Ernest Lau on 04/29/15 in support of Lau E, Wilson C, Ashfield K, McNair W, McEneany D, Roberts M, Large Capacity LiMnO2 Batteries Extended CRTD Longevity in Clinical Use Compared to Smaller Capacity LiSVO Batteries Over 6 Years. Presented at HRS 2015. Medtronic = 62 patients, Boston Scientific = 27 patients, St. Jude = 66 patients. Five-year survival rate calculated using device replacements for battery depletion as indicated by ERI.
- von Gunten S, Schaer BA, Yap SC, Szili-Torok T, Kühne M, Sticherling C, Osswald S, Theuns DA. Longevity of implantable cardioverter defibrillators: a comparison among manufacturers and over time. Europace. 2015 Nov 25; . Epub 2015 Nov 25. Total patients = 3436.
- Alam MB, Munir MB, Rattan R, Adelstein E, Jain S, Saba S. Battery longevity from cardiac resynchronization therapy defibrillators: differences between manufacturers and discrepancies with published product performance reports. Europace 016;doi:10.1093/europace/euw044. First published online: 22-MAR-2016. Kaplan Meier curves depicting survival of CRT devices free from battery depletion by device manufacturer. Battery Longevity in Cardiac Medtronic = 416 patients, Boston Scientific = 173 patients, St. Jude Medical = 57patients. Previously evaluated these patients at a four-year survival rate calculated using device replacements for battery depletion as indicated by ERI. 2014; Europace (2014) 16,246-51.
- Shabanna Din, Shabanna, Mcgee, Rao, Archana, Wright, Jay D. Longevity of implantable cardioverter defibrillators: The impact of device manufacturer and device type on device longevity were assessed. Europace. 2015 Nov 25. Epub 2015 Nov 25. Total patients = 3436. Cardiostim Abstract 2016. Total patients = 1489
- Boston Scientific Data on File. February 2016.
- Boston Scientific CRM Product Performance Report. Data on file as of April 14, 2016
- Boston Scientific CRT-Ds with contemporary battery technology have 1.9 Ah. Medtronic CRT-Ds have 1.0 Ah. Amplia MRI CRT-D Surescan DTMB2D4 UK 2016 Manual pg 30. Viva XT CRT-D OUS Manual DTBA2D1 2013 page 28. DYNAGEN™ CRT-D, DYNAGEN™ X4 CRT-D, INOGEN™ CRT-D, INOGEN™ X4 CRT-D, ORIGEN™ CRT-D, ORIGEN™ X4 CRT-D Physician’s Technical Manual. Part Number: 259049-004 EN US 2014-04.
- Boston Scientific CRM Product Performance report. Data on file as of April 4th 2023
- Medtronic CRM Product Performance Report, DTBA2QQ Viva Quad XT CRT-D eSource date June 13, 2022
- Saba S, et al. Safety and Effectiveness of Multi-Site Pacing in Initial Non-Responders to Conventional Cardiac Resynchronization Therapy. LBCT presented at: 2021 Heart Rhythm Society; July 2021; Boston, MA.
- De Larochellière, I. Nault, J. Champagne, J. Sarrazin, F. Philippon, C. Steinberg, F. Molin, G. O'Hara, K. Roy, B. Plourde, and L. Blier Can J Cardiol. 2020 Oct; 36(10): S52
- ESC Guidance for Diagnosis and Management of CVD during the COVID-19 Pandemic: https://www.escardio.org/Education/COVID-19-and-Cardiology.
- 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC, European Heart Journal (2021) 00, 1-128 doi:10.1093/eurheartj/ehab368.
- 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA), European Heart Journal (2021) 00, 1-94 doi:10.1093/eurheartj/ehab364.
- Boehmer JP, Hariharan R, Devecchi FG, et al. A Multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail. 2017 Mar;5(3):216-25.
- Hernandez AF, Albert N, Allen L, et al. Multiple cardiac sensors for management of heart failure (MANAGE-HF) Phase I results. Abstract presented at: European Society of Heart Failure 2021 World Congress on Acute Heart Failure: June 29-July 1, 2021. Virtual.
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Information for use only in countries with applicable health authority registrations. Material not intended for use in France.
All cited trademarks are the property of their respective owners.
















