The Importance of Device Longevity Dr. Fredrik Gadler explains how device longevity is becoming increasingly important for patients and for the healthcare system.
Device Replacements Nearly Double the Infection Risk
In an independent study from Leiden University, of the 451 patients that underwent a device replacement, 9.1% had a complication or infection. Of those with complications, 66% experienced a major complication that required reoperation. Furthermore, Investigators found that replacement doubled the risk for pocket-related surgical reintervention.⁴
Device Replacements Infection Rates
The infection rate for new device implants is <1% whereas risk increases to 2.6 to 7% after device replacements.⁴,⁵
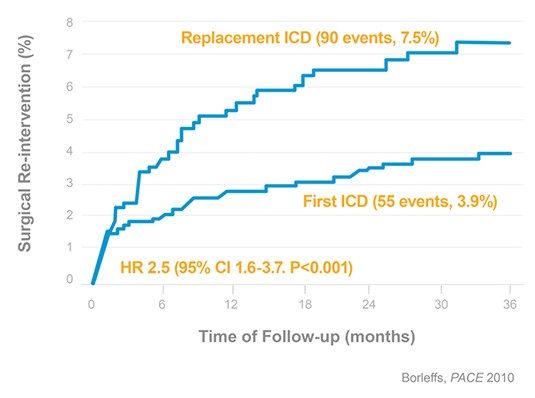
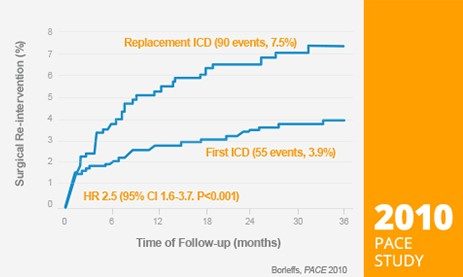
Doubled risk for pocket-related surgical re-interventions⁶
3,161 ICDs, 38 months follow-up
- 2.5 times increased risk for infection
- 1.7 times increases risk other complication
- Need for re-intervention increases with every consecutive replacement
References:
- Novation - Cardiovascular Watch: CRM Device Battery Life, May 2012 - https://www.novationco.com/other/apps/devicebattery/.
- Goldenberg I, Kutyifa V, Klein H, et. al. Survival with Cardiac-Resynchronization Therapy in Mild Heart Failure. NEJM 370;18: 1694-1701. Seven year survival relates to patients with left bundle branch block with QRS 130 ms, EF 30% NYHA Class I or II ischemic orNYHA Class II non-ischemic heart failure.
- Hauser. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. JACC 2005; 45; 2022-5.
- Gould P, et al. Outcome of Advisory Implantable Cardioverter-Defibrillator Replacement: One Year Follow-up. Heart Rhythm 2008; Vol. 5 #12: 1675-1681.
- Tarakji, Khaldoun G. et al. CIED infections: presentation, management and patient outcomes. Heart Rhythm; Aug 2010; 7:1043-1047
- Borleffs CJ, Thijssen J, De Bie MK, Van Rees JB, Van Welsenes GH, et al., Recurrent implantable cardioverter-defibrillator replacement is associated with an increasing risk of pocket-related complications. PACE 2010; 33: 1013-1019.
CAUTION:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Information for use only in countries with applicable health authority registrations. Material not intended for use in France.
All cited trademarks are the property of their respective owners.
















