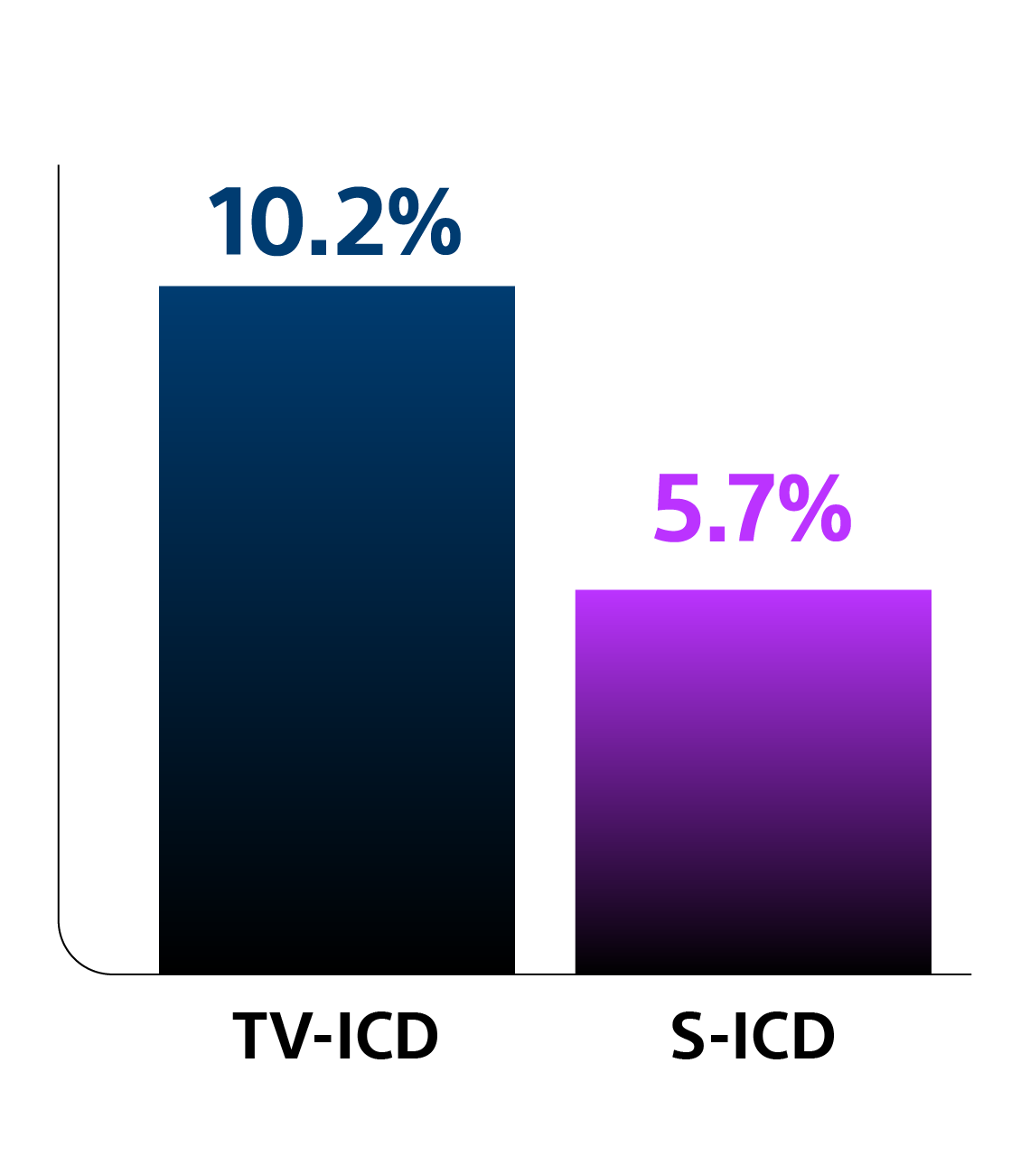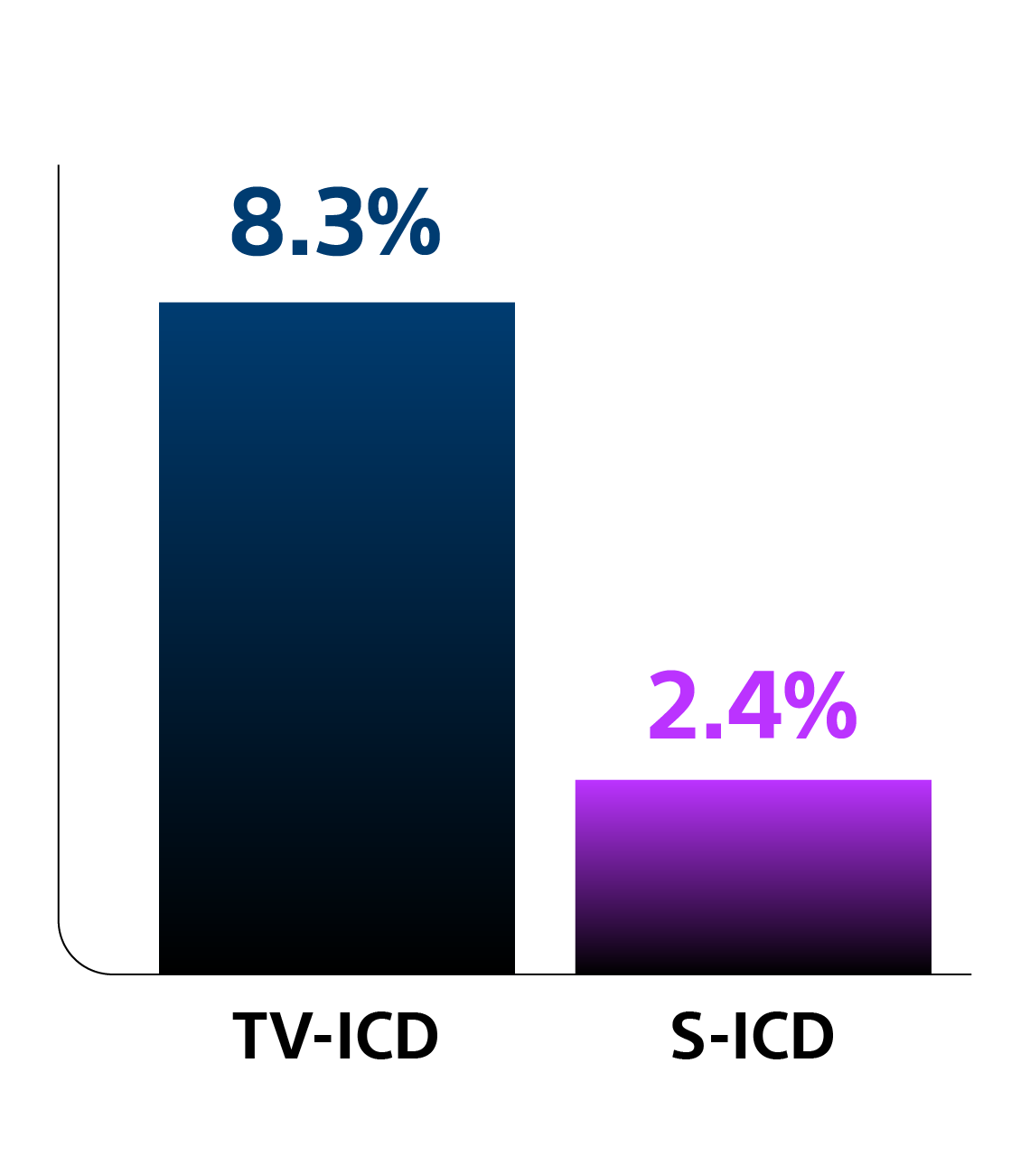PRAETORIAN Trial1
Head-to-head Trial: Subcutaneous Implantable Cardioverter Defibrillator (S-ICD) vs the Transvenous ICD (TV-ICD)
A prospective, randomised, controlled noninferiority trial comparing the S-ICD with the TV-ICD in patients with an ICD indication and no pacing requirement.1
Patient Population
•Total: N=849
•S-ICD group: n=426
•TV-ICD group: n=423
Key Results
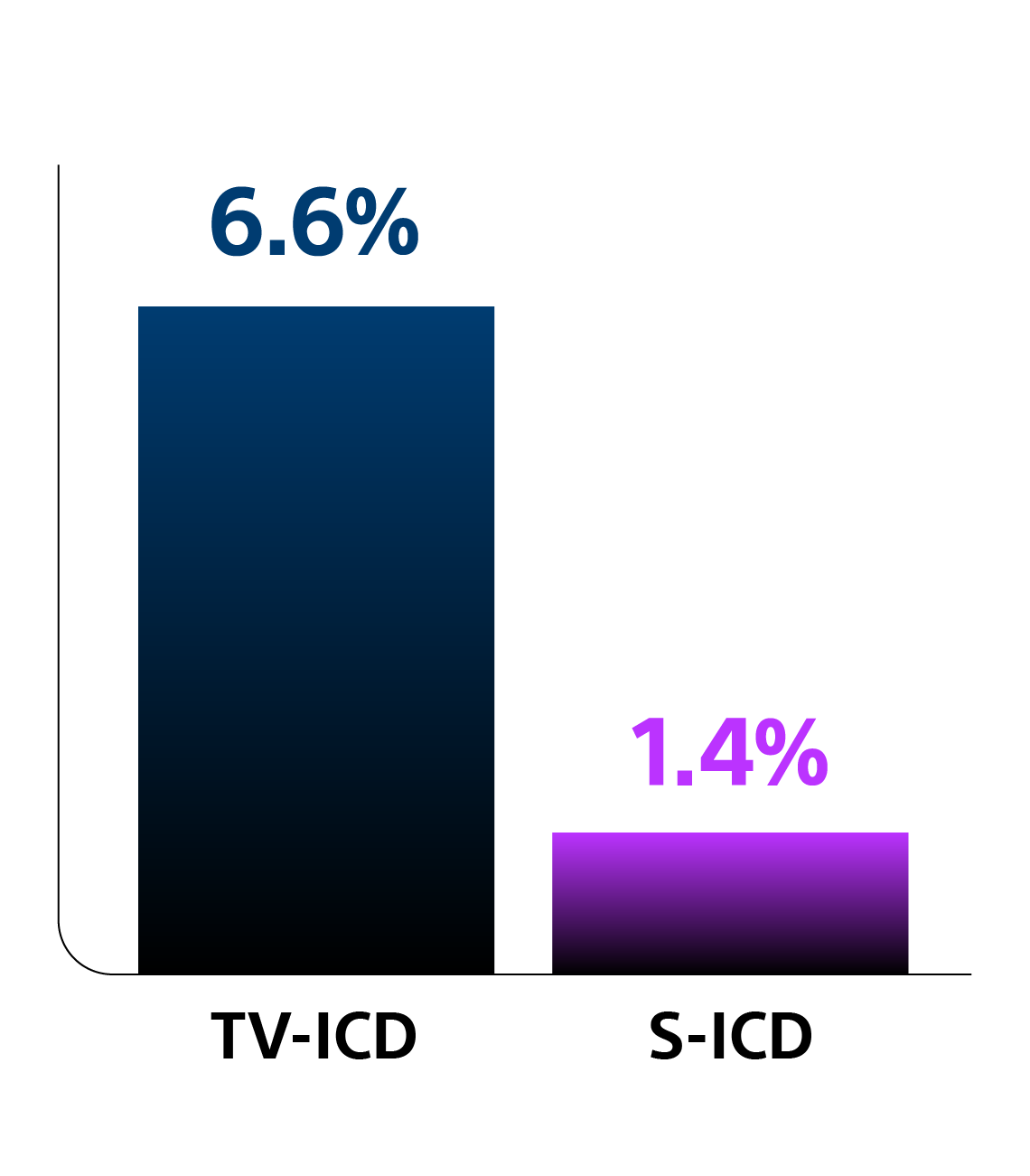
PRAETORIAN XL Trial2
A 4-year extension of PRAETORIAN, evaluating long-term outcomes over 8 years in a subset of the original cohort.
Patient Population
•S-ICD group: n=263
•TV-ICD group: n=265
Key Results