EFFORTLESS Registry
Trial Design
The EFFORTLESS S-ICD Registry is a non-randomized, standard of care, multicenter registry designed to collect long term system-related, clinical, and patient reported outcome data from S-ICD implanted patients since June 2009.
- 1000 patients followed for 5 years post implant
- Includes both retrospective and prospective patients implanted since 2009
- Centers to be included from all current commercial countries
- QOL sub-study (not yet reported at the time of this interim analysis)
Inclusion Criteria
- ≥ 18yrs at time of consent*
- Eligible for implantation of an S-ICD System per local clinical guidelines or currently implanted with a S-ICD System
Exclusion Criteria
- Participation in any other investigational study that may interfere with interpretation of the study results
- Pace-terminable VT
- Previously implanted unipolar pacemakers
Definition of Complications
- Type I: caused by the S-ICD System
- Type II: caused by the S-ICD System user’s manual or labeling of the S-ICD System
- Type III: not caused by the S-ICD System but would not have occurred in the absence of the implanted S-ICD System
The interim results were published online in the European Heart Journal in March 2014.
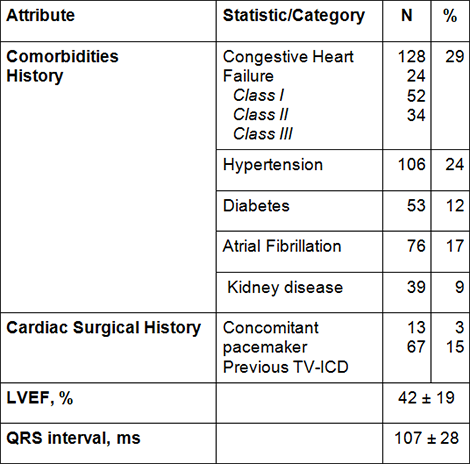
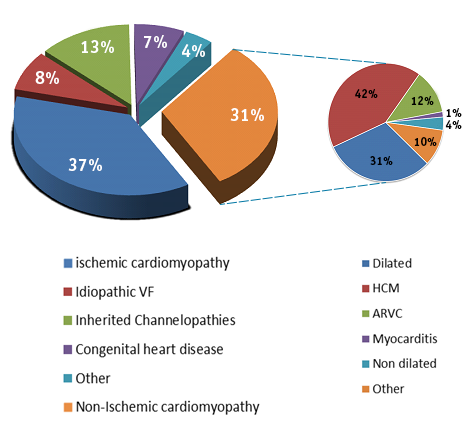
Demographics
Trial Results
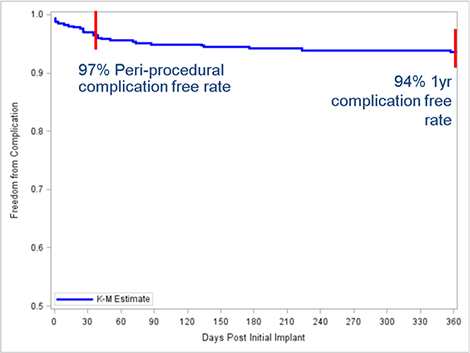
Complication-Free Rate
Complications were defined as clinical events where mitigation required an invasive procedure. There were no electrode failures, no systemic blood infections related to the S-ICD System or Procedure and no endocarditis. Complications occurred in 29 patients giving a patient complication event rate of 6.4% at an average follow-up of 558 days. In 14 patients (3%) complications occurred in the first 30-day post implant, for a peri-operative complication-free rate of 97%.
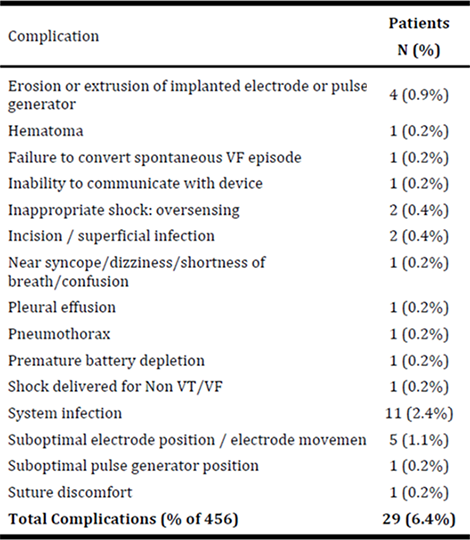
Implant conversion testing
Of 393 patients with complete data, in all but 1 patient VT/VF was successfully converted (99.7%). Implant testing with shock energy of ≤65J was successful in 95% of patients.
Spontaneous Conversion Efficacy
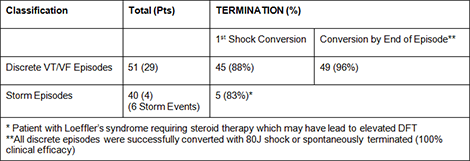
Time to Therapy
| Included | 15.1± 3.7 sec. |
| Spontaneous | 17.5 ± 4.4 |
- Therapy withheld in 13% of patients and 46% of stored episodes due to longer time to therapy
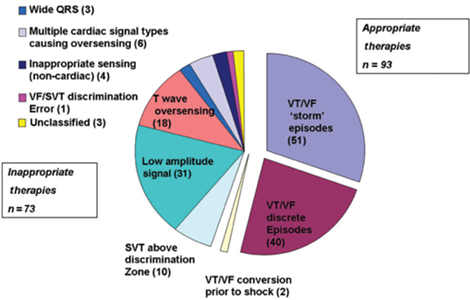
Inappropriate Therapy
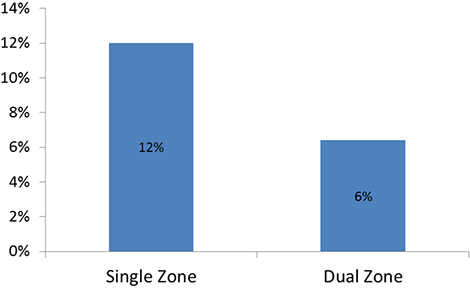
Inappropriate shocks were recorded in 32 patients over an average follow-up of 18 months (360 day inappropriate shock rate of 7%).
Dual zone programming (patients programmed with a conditional shock zone that applies SVT discrimination algorithms) had a 6.4% inappropriate shock rate (23/357) at an average follow-up of 558 days, while single zone programming had a 12% rate (9/74) [P = 0.09].
Inappropriate Shocks by Programming
One inappropriate therapy due to VF/SVT discrimination error within the conditional zone.