MADIT-CRT
The Multicenter Automatic Defibrillator Implantation Trial – Cardiac Resynchronization Therapy (MADIT-CRT)
The Multicenter Automatic Defibrillator Implantation Trial – Cardiac Resynchronization Therapy (MADIT-CRT)
Status: Study met its primary endpoint June 2009
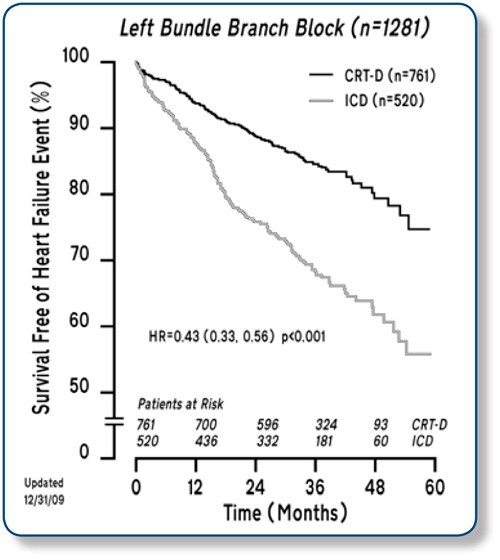
Further analysis shows:
- These impressive results were driven by a 35% relative reduction in the risk of all-cause mortality (p = 0.048) and a 63% relative reduction in the risk of first heart failure events (p < 0.001).1
- Notably, in the MADIT-CRT LBBB subgroup, 82% of the first heart failure events involved hospitalization.
- The Kaplan-Meier analysis reveals that improvement in the CRT-D arm is seen remarkably early — within a few months after implant — and continues for the duration of the trial.
- Delaying that first heart failure event in Class I and II patients is critical — in the MADIT-CRT trial, patients were 8 times more likely to have a recurring HF event after the initial event.
- Early CRT intervention reduces the relative risk of recurrent heart failure events by 43% in high-risk,3 NYHA Class I and II patients with LBBB.
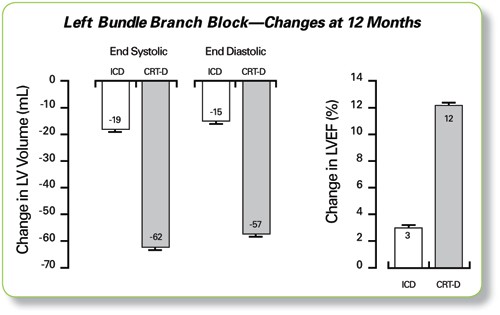
In an exploratory analysis that is hypothesis-generating only, the MADIT-CRT LBBB subgroup experienced a 12% increase in LVEF with a CRT-D, as compared to a 3% increase with an ICD. Also, patients with a CRT-D experienced a greater reduction in left ventricular volumes.
* CRT therapy was ON during echographic measurements and may have influenced the results.
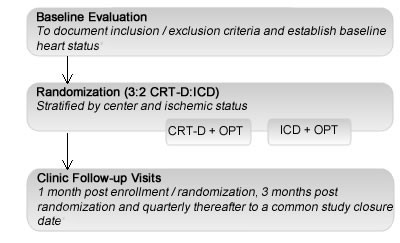
MADIT-CRT is the largest randomized NYHA Class I / II CRT-D trial to date
• Full Trial Population: 1820 patients
• LBBB Population: 1281 patients
Inclusion Criteria:
- Male and female
- Over 21 years of age
- NYHA functional Class II, non-ischemic or ischemic cardiomyopathy or
- NYHA functional Class I, ischemic cardiomyopathy and
- Left ventricular dysfunction (LVEF ≤ 0.30) and
- Prolonged intraventricular conduction (QRS duration ≥ 130 ms) and
- Optimal HF pharmacologic therapy (OPT)
110 centers in 14 countries
- To determine whether CRT-D in high-risk* heart failure patients will reduce the combined endpoint of all cause mortality or HF intervention when compared to ICD therapy.
- Primary Endpoint: all-cause mortality or heart failure intervention, whichever occurs first.
- MADIT-CRT is an important continuation of Boston Scientific’s sponsorship of landmark clinical trials such as MADIT, MADIT II, and COMPANION. MADIT-CRT may prove that early intervention with CRT can slow the progression of heart failure.














