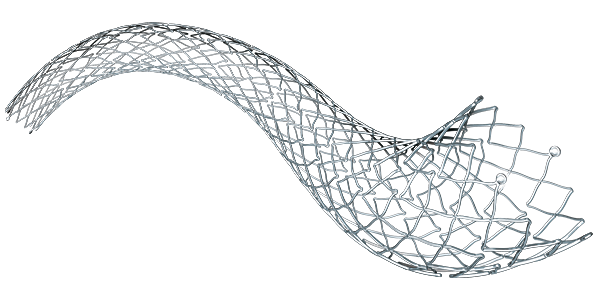
Epic™
Vascular Self-Expanding Stent System
Ideal for the Iliac: The Epic Stent is a self-expanding Nitinol stent designed to sustain vessel patency, while providing enhanced visibility and accuracy during placement.
Explore
Product Details
Epic Stent System provides exactly the right balance
Exceptional Flexibility
|
Balanced Radial Force
|
Excellent Deployment Accuracy
|
Fracture Resistance
|
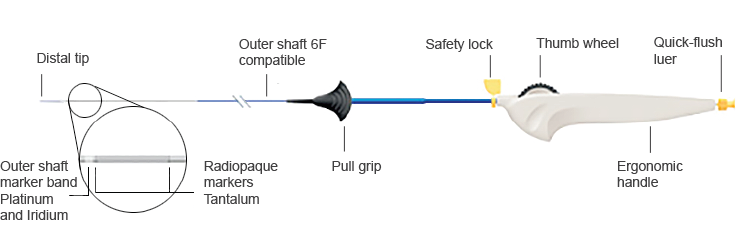
Product Specifications
| Stent Diameters | 5 to 14 mm |
| Stent Lengths | 20, 30, 40, 50, 60, 70, 80, 100, 120 mm |
| Catheter Lengths | 75, 120 cm |
| Guide Wire System | 0.035" OTW |
| Introducer Sheath Compatibility | 6 F across all sizes |
| Catheter Nominal O.D. | 0.079" to 0.077" (distal to proximal) |
Epic Stent Size Matrix
| 6 F Compatibility Across All Sizes | ||||||||||
| Length (mm) | ||||||||||
| Diameter (mm) | ||||||||||
| 20 | 30 | 40 | 50 | 60 | 70 | 80 | 100 | 120 | ||
| 5 | ||||||||||
| 6 | ||||||||||
| 7 | ||||||||||
| 8 | ||||||||||
| 9 | ||||||||||
| 10 | ||||||||||
| 12 | ||||||||||
| 14 | ||||||||||
| Catheter lengths: 75 cm, 120 cm Guidewire system: 0.035" OTW |
||||||||||
Featured clinical data
ORION US IDE Clinical Trial
3 year results
- 0 amputations at 3 years (106 subjects)
- 89.9% freedom from TLR at 3 years (143 Iesions)
Composite Success Rates
- 100% TechnicaI Success (Per lesion) (166/166)
- 99.2% Procedural Success (Per subject) (124/125)
- 95.3% Late Clinical Success at 12 months (Per subject) (101/106)
Lesions at risk
| All | 163.5 | 155 | 153 | 152.5 | 132 |
| A/B | 132.5 | 124 | 123 | 122.5 | 104 |
| C/D | 27 | 27 | 26 | 26 | 25 |
12-month data
- 0% Ml at 30 days (0/111)
- 3.4% MAE at 9-months (4/117)
- 0% MI in-hospital (0/111)
- 5.4% TVR at 12-months (6/111)
- 0% Amputation of index limb at 12-months (0/111)
Tools and Resources
-
 Dec 11, 2025
Dec 11, 2025MRI Compatibility for PI Products
Magnetic Resonance Imaging (MRI) Safety for Boston Scientific Peripheral Products PDF, 277.0 KB