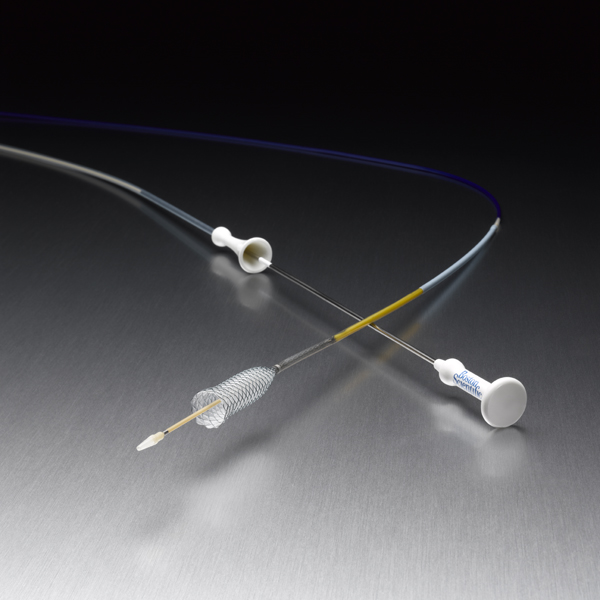
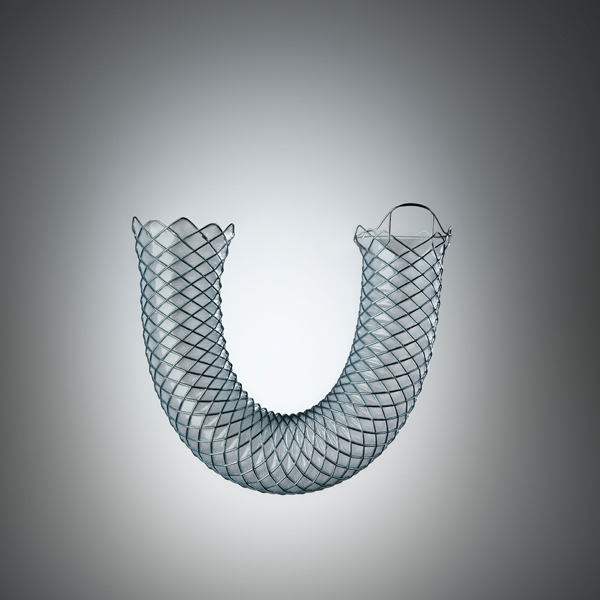
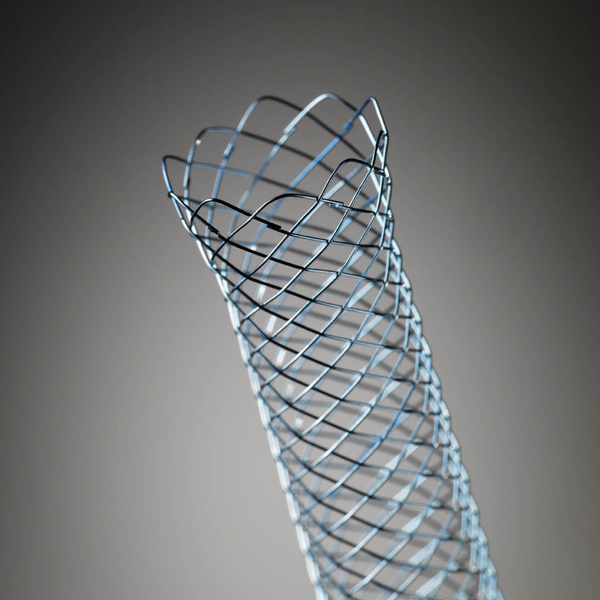
WallFlex™
Biliary RX Stent (including Benign Indication)
Two decades of metal stent development and demonstrated safety and effectiveness from multiple studies have helped make WallFlex Biliary Stents the most frequently implanted biliary metal stent throughout the U.S., Canada, and Europe.1
Key Resources
Explore
Ordering Information
WallFlex Biliary RX Stents
Fully covered, partially covered and uncovered WallFlex Biliary RX Stents are available in multiple sizes to accommodate different anatomical and clinical requirements. Below please find ordering information for WallFlex Biliary RX Stents. These stents may be used with short or long guidewires.

| Order Number | Diameter (mm) | Length (mm) | Catheter Diameter (Fr/mm) | Guidewire Diameter (in/mm) |
|---|---|---|---|---|
| M00574210 | 6 | 40 | 8.5/2.83 | .035/ .89 |
| M00574230 | 6 | 60 | 8.5/2.83 | .035/ .89 |
| M00574240 | 6 | 80 | 8.5/2.83 | .035/ .89 |
| M00570450 | 8 | 60 | 8.5 / 2.83 | .035 / .89 |
| M00570460 | 8 | 80 | 8.5 / 2.83 | .035 / .89 |
| M00576800 | 8 | 100 | 9.0 / 3.00 | .035 / .89 |
| M00576810 | 8 | 120 | 9.0 / 3.00 | .035 / .89 |
| M00570470 | 10 | 40 | 8.5 / 2.83 | .035 / .89 |
| M00570480 | 10 | 60 | 8.5 / 2.83 | .035 / .89 |
| M00570490 | 10 | 80 | 8.5 / 2.83 | .035 / .89 |
| M00576820 | 10 | 100 | 9.0 / 3.00 | .035 / .89 |
| M00576830 | 10 | 120 | 9.0 / 3.00 | .035 / .89 |
* Compatible with Boston Scientific .035" guidewires, including DreamwireTM Guidewire.
* Through non-clinical testing, the WallFlex Biliary RX Stent has been shown to be MR Conditional (poses no known hazards under specified conditions) when used according to conditions described in the Directions for Use.

| Order Number | Diameter (mm) | Length (mm) | Catheter Diameter (Fr/mm) | Guidewire Diameter (in/mm) |
|---|---|---|---|---|
| M00570700 | 8 | 60 | 8.5 / 2.83 | .035 / .89 |
| M00570710 | 8 | 80 | 8.5 / 2.83 | .035 / .89 |
| M00576740 | 8 | 100 | 9.0 / 3.00 | .035 / .89 |
| M00576750 | 8 | 120 | 9.0 / 3.00 | .035 / .89 |
| M00570720 | 10 | 40 | 8.5 / 2.83 | .035 / .89 |
| M00570730 | 10 | 60 | 8.5 / 2.83 | .035 / .89 |
| M00570740 | 10 | 80 | 8.5 / 2.83 | .035 / .89 |
| M00576760 | 10 | 100 | 9.0 / 3.00 | .035 / .89 |
| M00576770 | 10 | 120 | 9.0 / 3.00 | .035 / .89 |
* Compatible with Boston Scientific .035" guidewires, including DreamwireTM Guidewire.
* Through non-clinical testing, the WallFlex Biliary RX Stent has been shown to be MR Conditional (poses no known hazards under specified conditions) when used according to conditions described in the Directions for Use.

| Order Number | Diameter (mm) | Length (mm) | Catheter Diameter (Fr/mm) | Guidewire Diameter (in/mm) |
|---|---|---|---|---|
| M00570600 | 8 | 40 | 8.0 / 2.67 | .035 / .89 |
| M00570610 | 8 | 60 | 8.0 / 2.67 | .035 / .89 |
| M00570620 | 8 | 80 | 8.0 / 2.67 | .035 / .89 |
| M00570630 | 8 | 100 | 8.0 / 2.67 | .035 / .89 |
| M00576780 | 8 | 120 | 8.0 / 2.67 | .035 / .89 |
| M00570890 | 10 | 40 | 8.0 / 2.67 | .035 / .89 |
| M00570640 | 10 | 60 | 8.0 / 2.67 | .035 / .89 |
| M00570650 | 10 | 80 | 8.0 / 2.67 | .035 / .89 |
| M00570660 | 10 | 100 | 8.0 / 2.67 | .035 / .89 |
| M00576790 | 10 | 120 | 8.0 / 2.67 | .035 / .89 |
* Compatible with Boston Scientific .035" guidewires, including Dreamwire® Guidewire.
* Through non-clinical testing, the WallFlex Biliary RX Stent has been shown to be MR Conditional (poses no known hazards under specified conditions) when used according to conditions described in the Directions for Use.
Case Studies
Results from this study are presented by Jin Ming, MD, Beijing, China
Disclaimer
1. Press Release - August 9, 2011, Boston Scientific's WallFlex™ Biliary RX Stent Receives Canadian Approval for Expanded Indication
All trademarks are the property of their respective owners. CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings and instructions for use can be found in the product labelling supplied with each device. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France. Product available in the European Economic Area (EEA) only. Please check availability with your local sales representative or customer service.