Catheters: Ablation
All Specialties (-)
-
BLAZER PRIME™ Temperature Ablation Catheter
Intuitively engineered construction combined with our trusted BLAZER™ Catheter platform provides an ablation catheter designed to function as a physical extension of your hand. Manipulation of the handle translates predictably into movement of the catheter tip. Control and durability provide procedural confidence.
- Electrophysiology
-
BLAZER™ II Temperature Ablation Catheter Family
Exceptional Clinical Results
- Electrophysiology
-
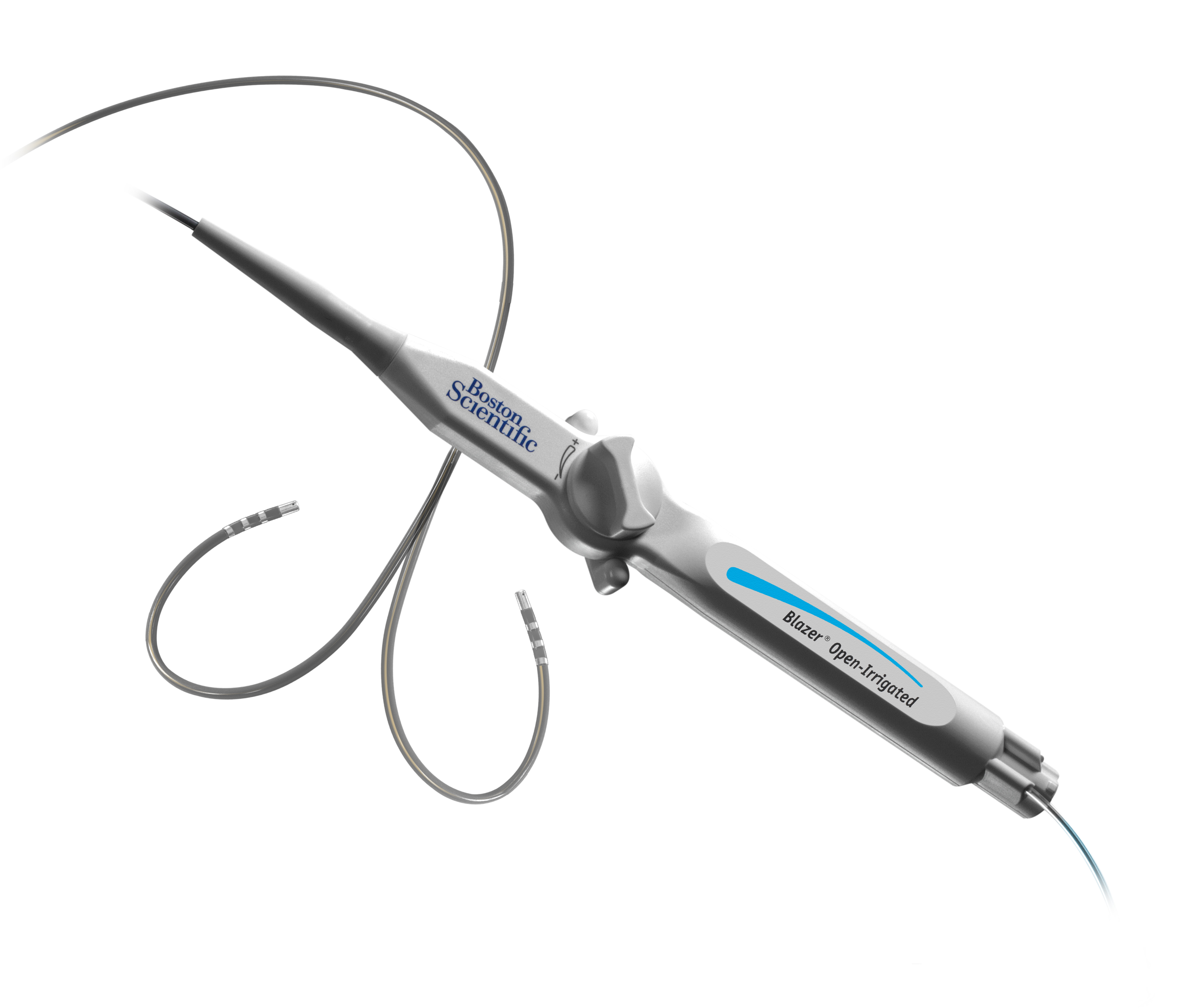
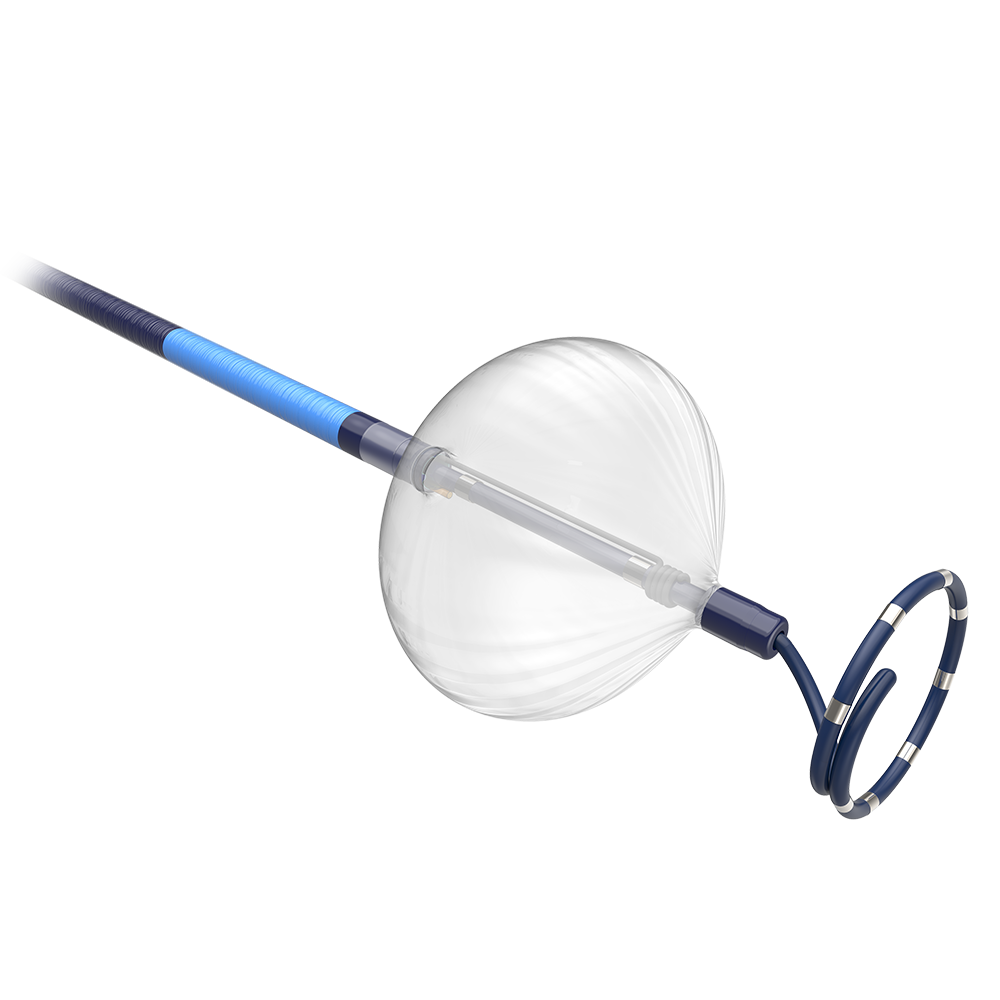
Blazer™ Open-Irrigated Ablation Catheter
The Blazer™ Open-Irrigated Ablation Catheter is the only catheter with a Total Tip Cooling™ Design which provides total uniform tip cooling internally, an optimized flow pattern with active washing and consistent cooling throughout RF delivery as well as a uniform external flow.
- Electrophysiology
-
FARADRIVE™ Steerable Sheath
FARADRIVE is a 13F steerable sheath specifically designed to navigate the FARAWAVETM Pulsed Field Ablation Catheter and the FARAWAVETM NAV Pulsed Field Ablation Catheter to the targeted cardiac anatomy. VersaCross ConnectTM Access Solution for FARADRIVE connects VersaCross technology with FARADRIVE for an integrated zero exchange left heart access workflow.
- Electrophysiology
-
FARAPOINT™ Pulsed Field Ablation Catheter
The FARAPOINT™ Pulsed Field Ablation (PFA) Catheter is designed to add focal precision to the FARAPULSE™ PFA Platform, providing electrophysiologists with a nav-enabled PFA catheter solution to create both focal and linear-shaped lesions in one tool.
- Electrophysiology
-
FARAPULSE™ Pulsed Field Ablation System
The most-proven PFA system transforming treatment for Afib
FARAPULSE Pulsed Field Ablation (PFA) is a non-thermal mechanism of ablation, delivering durable and effective lesions while reducing many traditional risks associated with thermal ablation. It has been used to treat 500,000 patients, positively transforming patient outcomes and clinical practice.- Electrophysiology
-
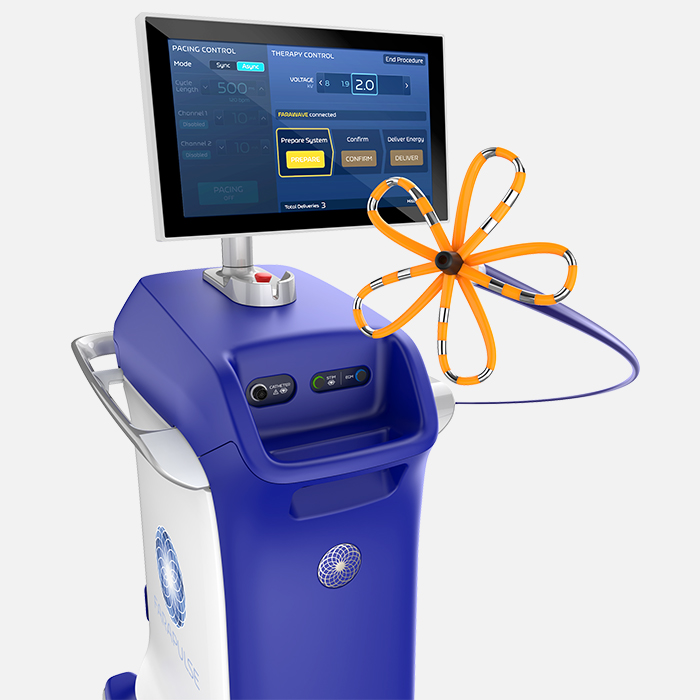
FARASTAR™ Pulsed Field Ablation Generator
The FARASTAR Pulsed Field Ablation (PFA) Generator generates and delivers a proprietary and biphasic waveform through the FARAWAVETM Pulsed Field Ablation Catheter and the FARAWAVETM NAV Pulsed Field Ablation Catheter.
- Electrophysiology
-
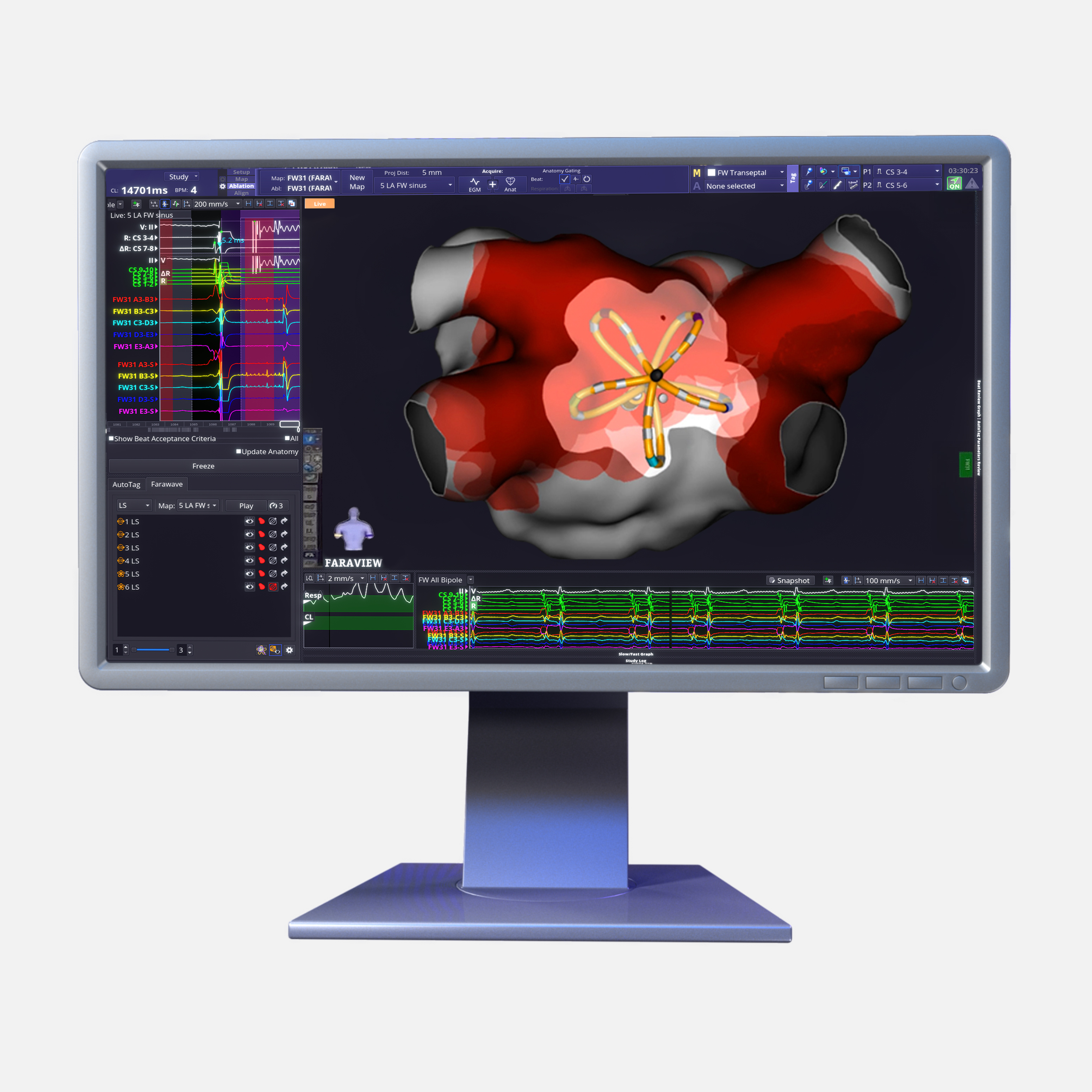
FARAVIEW™ Software Module
The FARAVIEW Software Module, seamlessly integrated with the OPAL HDxTM Mapping System, is an advanced cardiac mapping solution that dynamically visualises the size, shape, and rotation of the FARAWAVETM NAV Pulsed Field Ablation (PFA) Catheter.
- Electrophysiology
-
FARAWAVE™ NAV Pulsed Field Ablation Catheter
The FARAWAVE NAV Pulsed Field Ablation (PFA) Catheter includes magnetic tracking for optimal cardiac mapping integration with the FARAVIEWTM Software Module on the OPAL HDxTM Mapping System. This 5-spline over-the-wire single-catheter solution enables fully integrated cardiac mapping and PFA therapy delivery while minimising the need for additional exchanges.
- Electrophysiology
-
FARAWAVE™ Pulsed Field Ablation Catheter
FARAWAVE is an over-the-wire pulsed field ablation (PFA) catheter with two form factors—basket and flower—designed to effectively ablate a wide range of unique patient anatomies. It enables pulmonary vein isolation (PVI) in patients with paroxysmal atrial fibrillation (AF) and supports both PVI and posterior wall ablation (PWA) in patients with persistent AF (PersAF).
- Electrophysiology
-
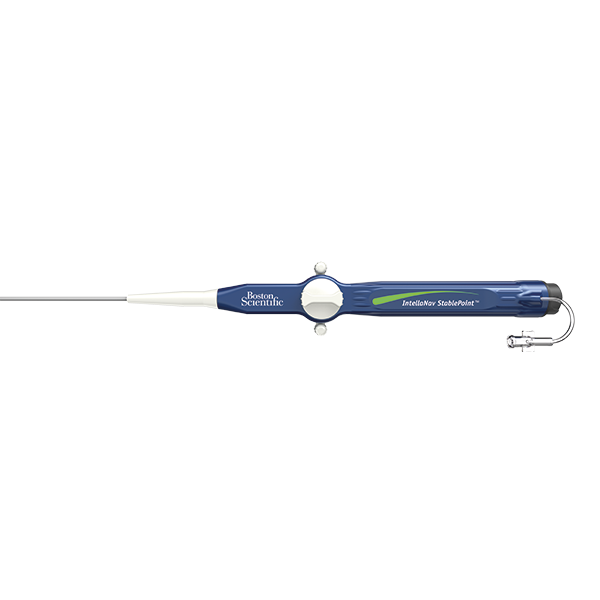
INTELLANAV STABLEPOINT™ Ablation Catheter
INTELLANAV STABLEPOINT™ Ablation Catheter, enabled with DIRECTSENSE™ Technology, combines the power of contact force and local impedance to give you dynamic insights at and below the cardiac tissue surface. Unlike any other technology, this integrated solution, available exclusively via OPAL HDx™, enables you to diagnose, ablate, and validate with more critical information than ever before.
- Electrophysiology
-
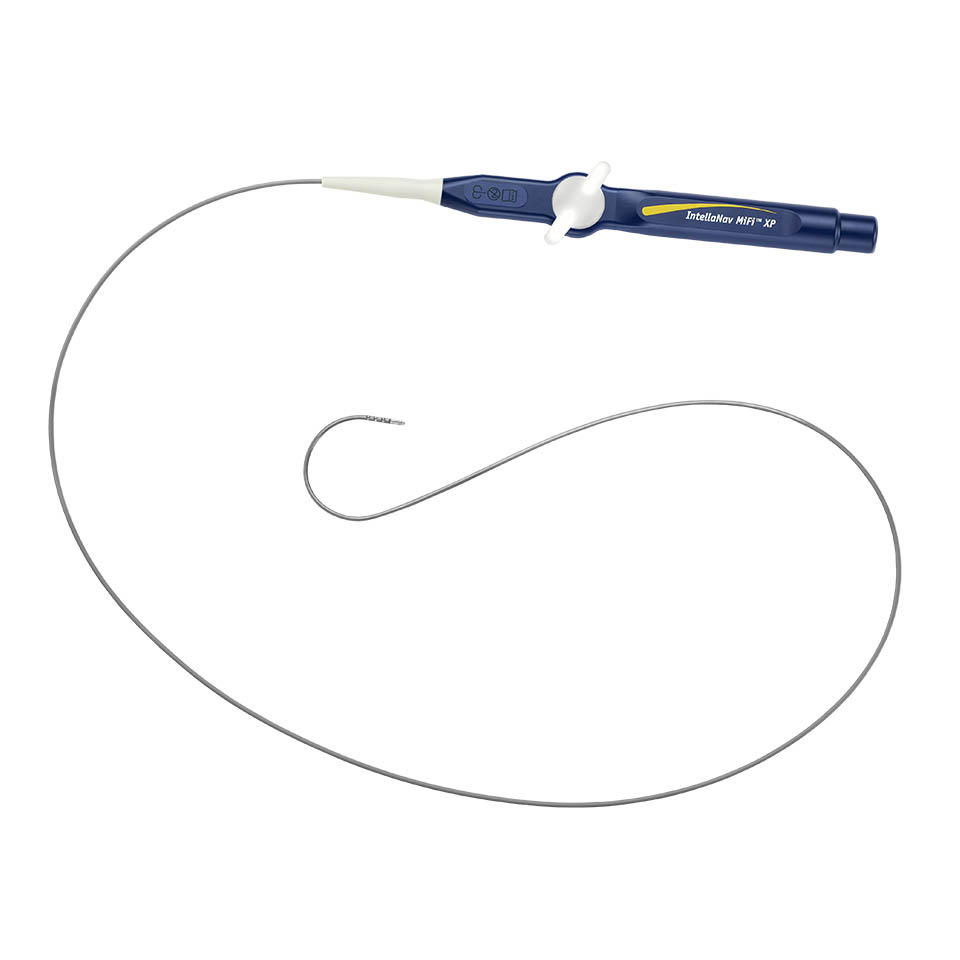
INTELLANAV MIFI™ XP Temperature Ablation Catheter Family
The INTELLANAV MIFI™ XP catheters encompass the proven performance of the BLAZER™ XP platform and the unparalleled clarity of MIFI technology in our magnetically tracked ablation catheters. These catheters unlock the magnetic tracking performance of the OPAL HDx™ Mapping System, enabling increased accuracy, clarity, and performance.1
- Electrophysiology
-
INTELLANAV™ ST Ablation Catheter
Combining the proven performance of the BLAZER™ platform with a navigation-enabled 4 mm solid tip, the INTELLANAV™ ST Ablation Catheter offers smaller, more precise ablation for increased accuracy, efficiency and performance in OPAL HDx™ Mapping System procedures.
- Electrophysiology
-
INTELLANAV™ MIFI OPEN-IRRIGATED Ablation Catheter
The INTELLANAV MIFI Open Irrigated Catheter brings together our proprietary Mini-Electrode technology with the Total Tip Cooling design and the IntellaNav magnetic tracking for Unparalleled Clarity, Cool Performance and Confident Navigation in OPAL HDx™ Mapping System procedures
- Electrophysiology
-
INTELLANAV™ OPEN-IRRIGATED Ablation Catheter
The INTELLANAV™ OPEN-IRRIGATED catheter encompasses the elegant Total Tip Cooling™ design and the familiarity and proven performance of the Blazer platform, now enhanced with INTELLANAV magnetic tracking technology for cool performance and confident navigation in OPAL HDx™ Mapping System procedures.
- Electrophysiology
-
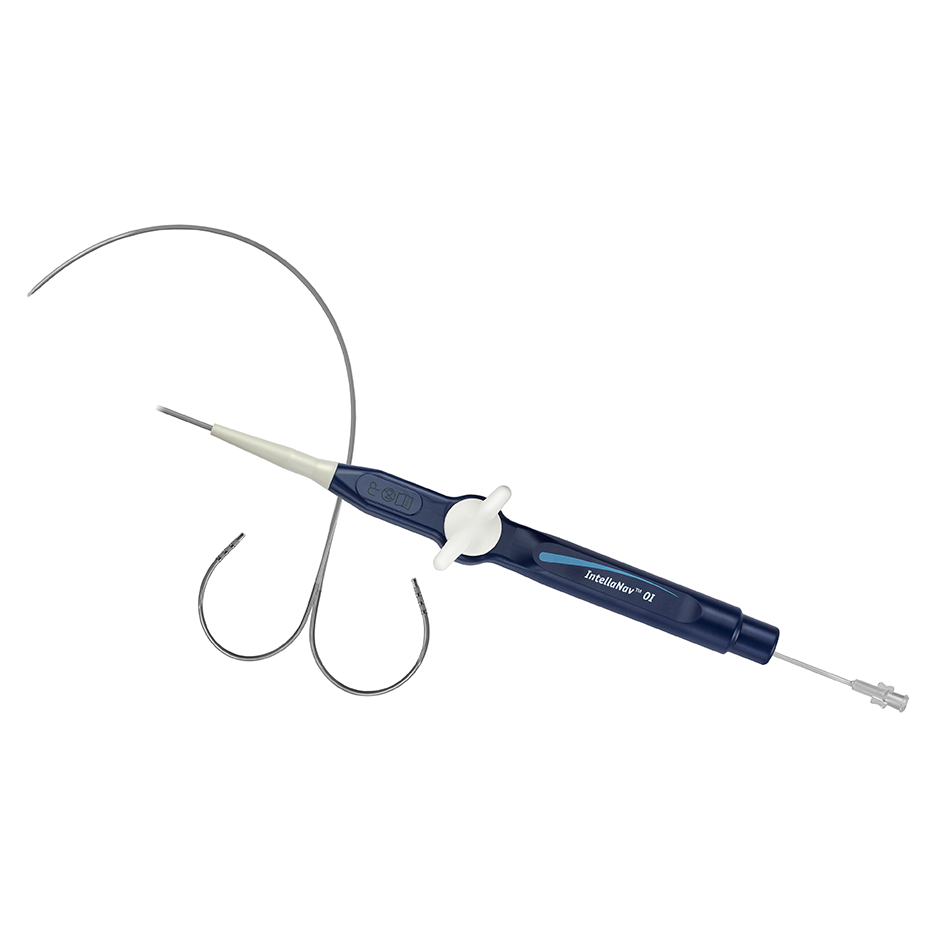
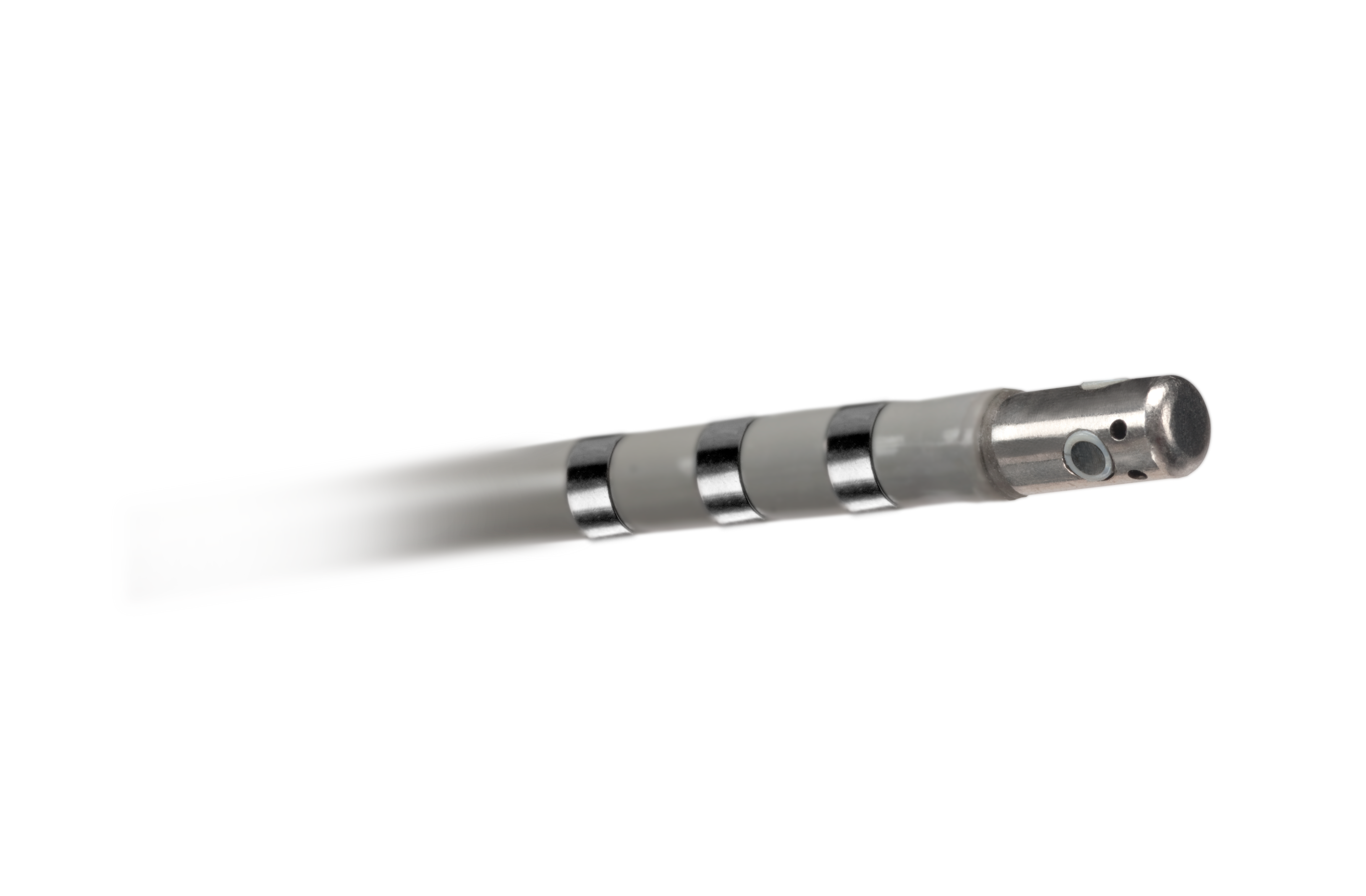
INTELLATIP MIFI™ Open-Irrigated (OI) Multi-Dimensional Ablation Catheter
The INTELLATIP MIFI™ OI Ablation Catheter delivers the critical, multi-dimensional information you need to confidently diagnose and treat complex cardiac arrhythmias and provide the true picture of exactly what is happening at the tip of the ablation catheter in real-time.
- Electrophysiology
-
POLARx™ Cryoablation System
The POLARx Cryoablation System has been designed to retain the best of the established approach and performance of cryoablation, then goes a step further to implement physician-driven changes to address well-documented limitations.
- Electrophysiology