Ranger™
Paclitaxel-Coated PTA Balloon Catheter
Ranger™ DCB is built on the physician-preferred Sterling™ 0.014”/0.018” balloon platform with a low entry profile.¹ It is design for highly efficient drug transfer to the lesion.²⁻⁴
Key Resources
Explore
- Boston Scientific Data on File. Ranger Catheter Competitive Testing Report, 92517674. Measurements from 6 x 120 devices for Ranger DCB, Lutonix™ 035.
- Sachar R, et al. RANGER II SFA PK Substudy. 2019.
- Steiner S, et al. COMPARE: prospective, randomized, non-inferiority trial of high- vs. low-dose paclitaxel drug-coated balloons for femoropopliteal interventions. Eur Heart J. 2020;41:2541–2552.
- Sachar R, et al. RANGER II SFA Investigators. 1-year results from the RANGER II SFA randomized trial of the Ranger drugcoated balloon. JACC Cardiovasc Interv. 2021;10:1123–1133.
Product Details
 |
Exceptional OutcomesRanger demonstrated consistent results with nearly 90% patency at 12-months in the RANGER II SFA and COMPARE Randomized Controlled Trials1. |
 |
Effortless DeliverabilityBuilt on the market-leading .018” Sterling balloon platform2 with .014”/.018" guidewire compatibility, Ranger has the lowest tip entry profile.3 |
 |
Efficient Drug TransferRanger is a low dose DCB with a uniquely formulated TransPax coating that results in highly efficient drug transfer. This enables extremely targeted drug delivery, achieving nearly 90% primary patency1, with the least amount of downstream particulates4 and low systemic drug exposure to the patient.5 |
COMPARE Randomized Controlled Trial: 12-Month Results presented by Sabine Steiner, MD. LINC 2020. 12-Month Primary Endpoints: Binary Primary Patency = 83.0% for Ranger DCB and 81.5% for IN.PACT DCB (Pnon-inferiority <0.01). Freedom from Major Adverse Events = 91.0% for Ranger DCB and 92.6% for IN.PACT DCB (Pnon-inferiority <0.01).
Ranger II SFA Randomized Controlled Trial: 12-Month Results presented by Marianne Brodmann. LINC 2020. 12-Month Primary Endpoints: Binary Primary Patency = 82.9% for Ranger DCB and 66.3% for PTA (p= 0.0017). Freedom from Major Adverse Events = 94.1% for Ranger DCB and 83.5% for PTA (Pnon-inferiority <0.0001).
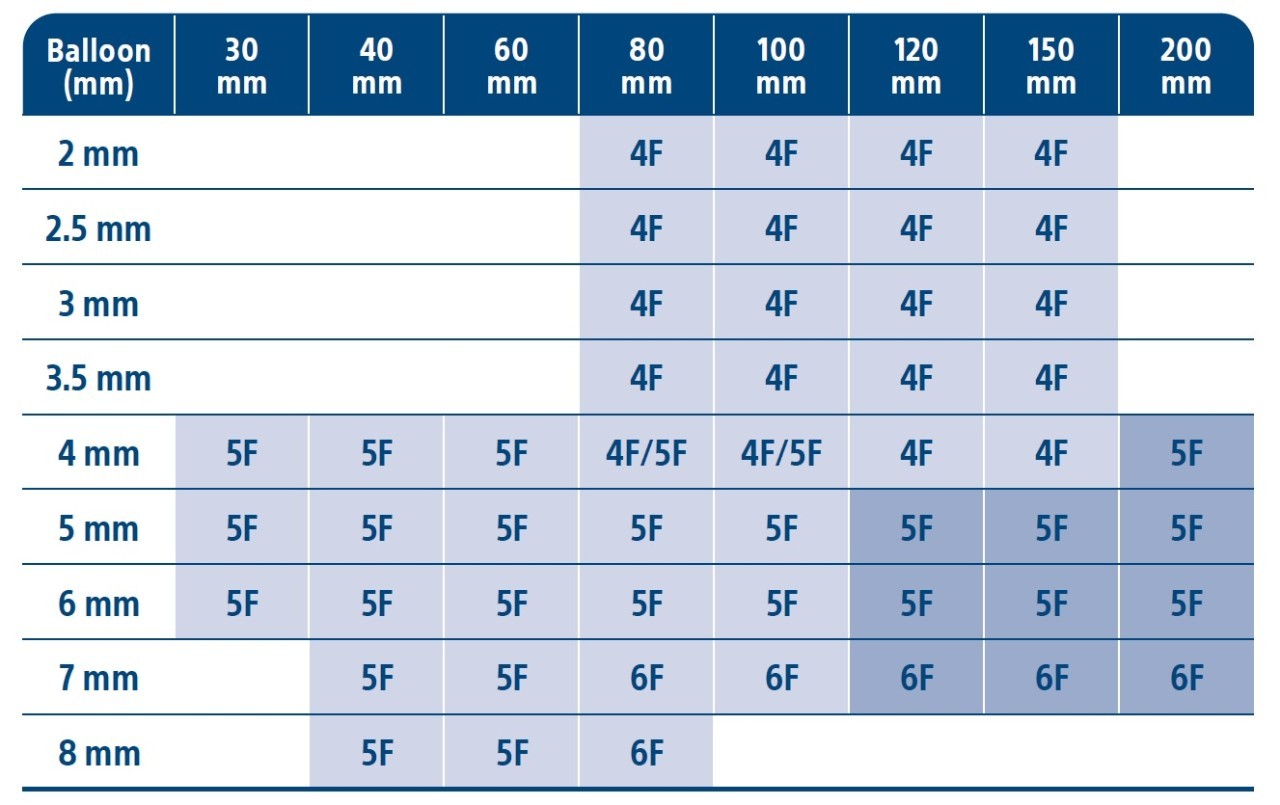
Ranger Size Matrix
- COMPARE Clinical Trial 12-Month Full Cohort Results presented by Sabine Steiner, MD. LINC 2020. K-M Primary Patency = 88.4%. RANGER II SFA Pivotal Trial 12-Month Results presented by Marianne Brodmann. LINC 2020. K-M Primary Patency = 89.8%.
- DRG data, CY 2019, 0.018” PTA Balloons.
- Boston Scientific Data on File. Ranger Catheter Competitive Testing Report, 92517674. Tip entry profile measurements taken from 6 x 120 devices.
- Gongora et al. Comparative Drug-Coated Balloon Study. JACC Cardiovasc Interv. 2015doi.org/10.1016/j.jcin.2015.03.020.
- RANGER II SFA PK Substudy presented by Ravish Sachar, MD. VIVA 2019.