The OPTALYSE PE Study is about optimum duration and dose of tPA with the acoustic pulse thrombolysis procedure for intermediate-high risk pulmonary embolism (PE).
Patients treated in the third arm at the OPTALYSE Trial (1 mg /rTPA / catheter / hour), obtained the best safety / efficacy balance, with improvement in quality of life, excervise tolerance and physical functioning at one year.
Tapson, Victor, et al., “A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial.” JACC: Cardiovascular Interventions Jul 2018, 11 (14) 1401-10
Clinical significance
Trial overview
- Prospective, randomized parallel-group
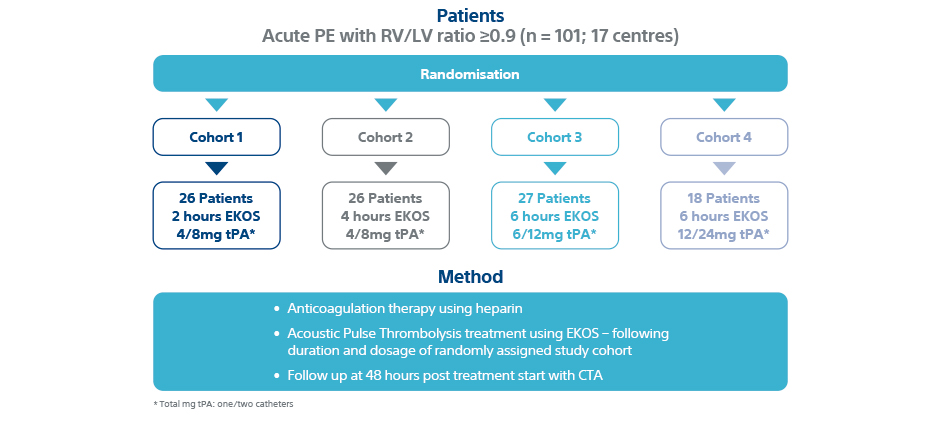
- 101 patients with acute intermediate-risk PE
- Patients randomly sorted into four separate low dose/short duration EKOS protocol cohorts
- 17 centres in US and Europe
- 23-26% reduction (p<0.0001) in RV/LV ratio 48 h from baseline across all cohorts
- Infusion time: 2, 4, 6 h Total dose: 4/8 -12/24 mg
- 4 major bleed (3%)
- 1 ICH
Patients
Key results
- RV/LV ratio continued to reduce after 48 hours.
- P value <0.0001 at 365 days.
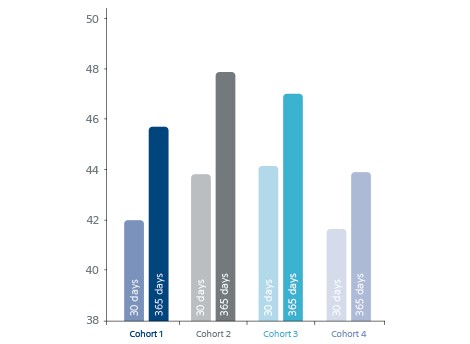
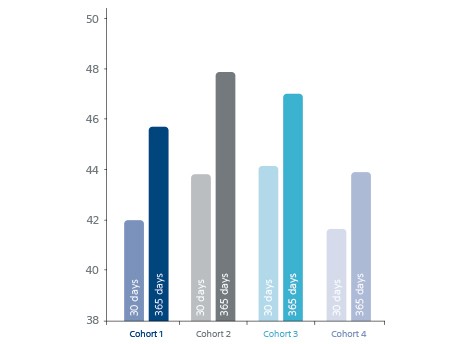
- Long-term OPTALYSE safety and efficacy at 365 days.
- PEmb-QoL score decreased for all cohorts.
- PROMIS-PF score increased for all cohorts.
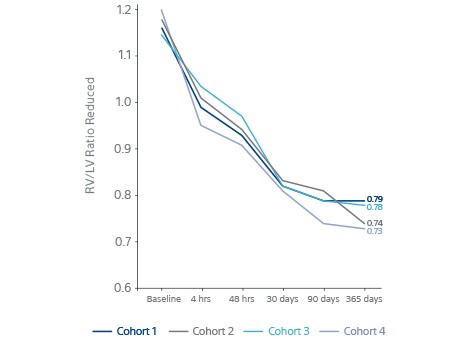
RV/LV Ratio Continued to reduce after 48 h P Value <0.0001 @365d
| Mortality | Recurrent PE | |
| OPTALYSE PE | 2% | 2% |
| PEITHO-AC | 8% | N/A |
| PEITHO-TNK | 10% | N/A |
| Baglin – AC | N/A | 3.70% |
Long-term OPTALYSE safety and efficacy at 365d
PEmb-QoL score decreased for all cohorts
PROMIS-PF score increased for all cohorts
Trial conclusions
The EKOS™ System’s very low dose and short duration regimens, in the OPTALYSE PE Trial, resulted in rapidly improved measures of right-heart function that were maintained for one year. Additionally, the favourable mortality rates, recurrent PE rates, and quality-of-life results demonstrate long-term benefits of EKOS therapy. This data further proves the PE clinical efficacy and safety of the OPTALYSE PE treatment protocols.
This study confirmed that EKOS™ was safe and effective with a lower dose tPA and shorter duration. OPTALYSE provided a proven patient protocol for the interventional treatment of PE. It also supplied long-term RV remodeling and QOL data to the EKOS™ data set.
Trial bibliography
2018 – OPTALYZE PE: Optimum Duration and Dose of tPA with the Acoustic Pulse Thrombolysis Procedure for intermediate high-risk pulmonary embolism.
Tapson, Victor, et al., “A Randomised Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial.” JACC: Cardiovascular Interventions Jul 2018, 11 (14) 1401-10.
https://pubmed.ncbi.nlm.nih.gov/30025734/
2020 – OPTALYSE PE: G. Piazza MD, MS et al., “One-Year Echocardiographic, Functional, and Quality of Life Outcomes After Ultrasound-Facilitated Catheter-Based Fibrinolysis for Pulmonary Embolism.” Cardiovascular Interventions Aug 2020, 13 (08), :e009012. doi: 10.1161/CIRCINTERVENTIONS.120.009012. Epub 2020 Aug 6. PMID: 32757658; PMCID: PMC7434215.
https://pubmed.ncbi.nlm.nih.gov/32757658/
Caution:
The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at www.IFU-BSCI.com. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France.
















