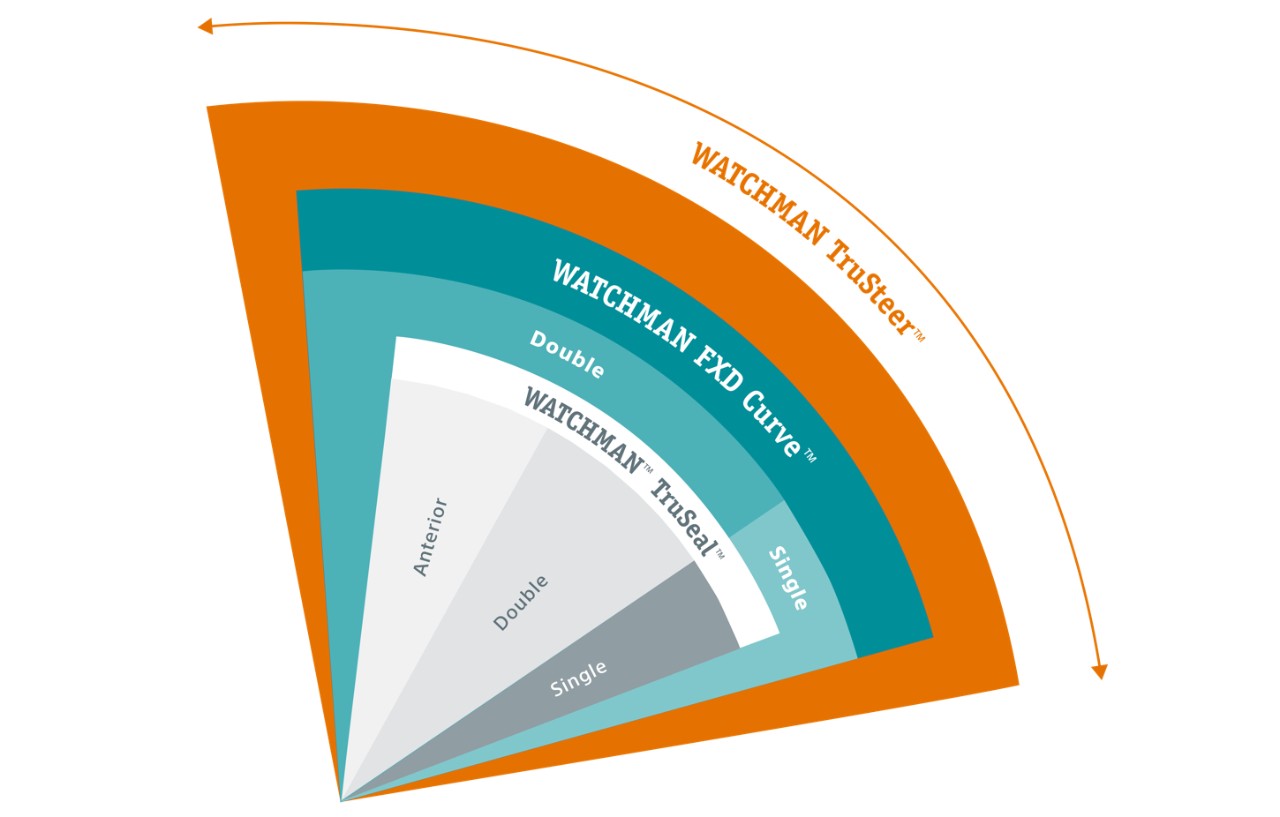
Precision for every anatomy
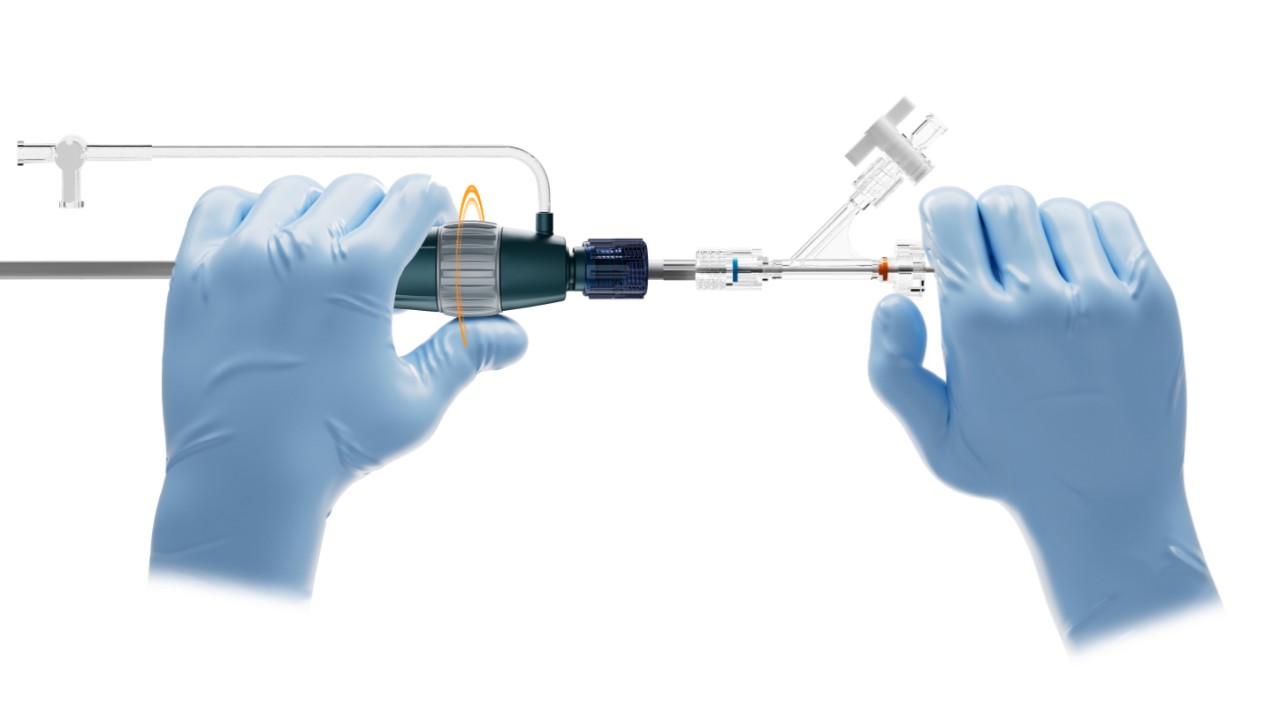
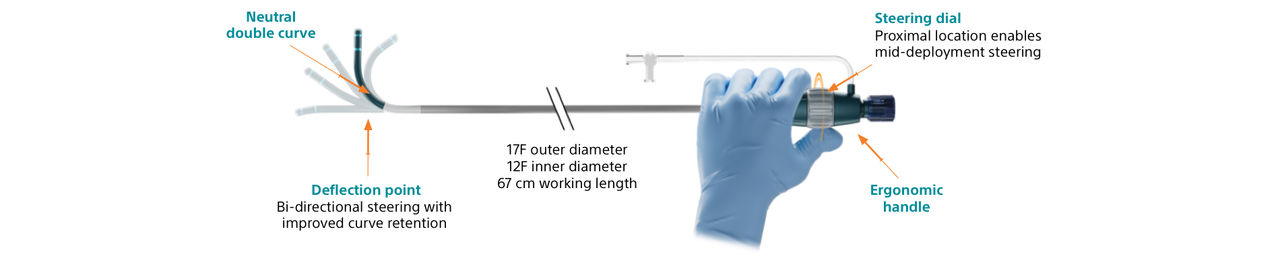
The WATCHMAN TruSteerTM Access System has bi-directional steering and a new ergonomic handle that allow you to:
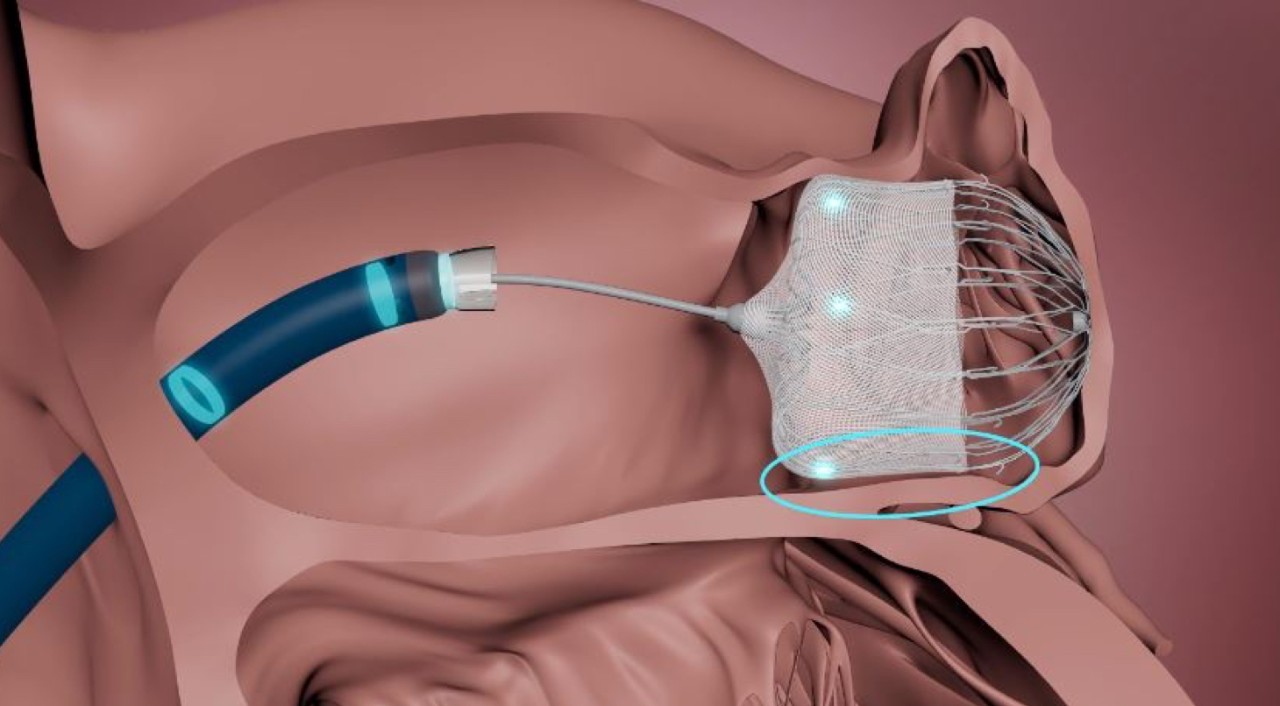
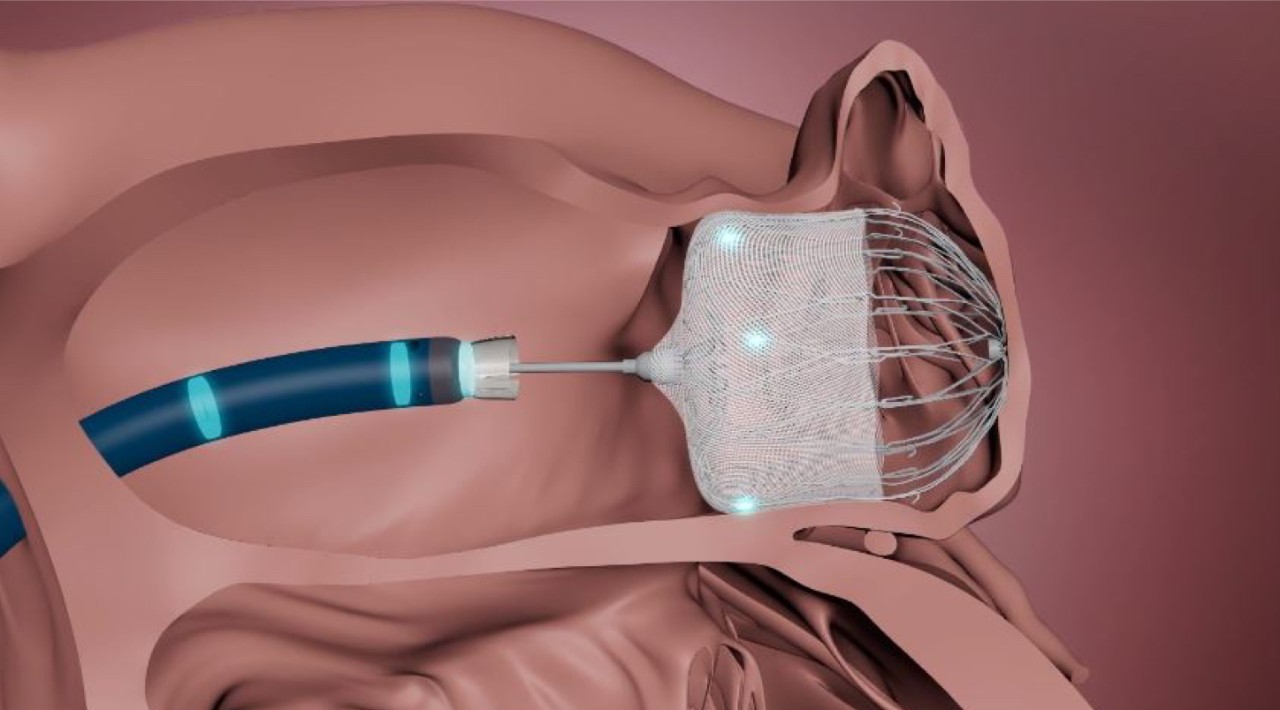
- Control WATCHMAN FLXTM Pro device deployment trajectory for optimized device positioning.
- Achieve a greater range of motion to reach the widest range of anatomies.
- Enable optimal coaxial tug test for more accurate device assessment prior to release.
WATCHMAN TruSteer: Advancing LAAC Access and Control


CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France. 2025 Copyright © Boston Scientific Corporation or its affiliates. All rights reserved.