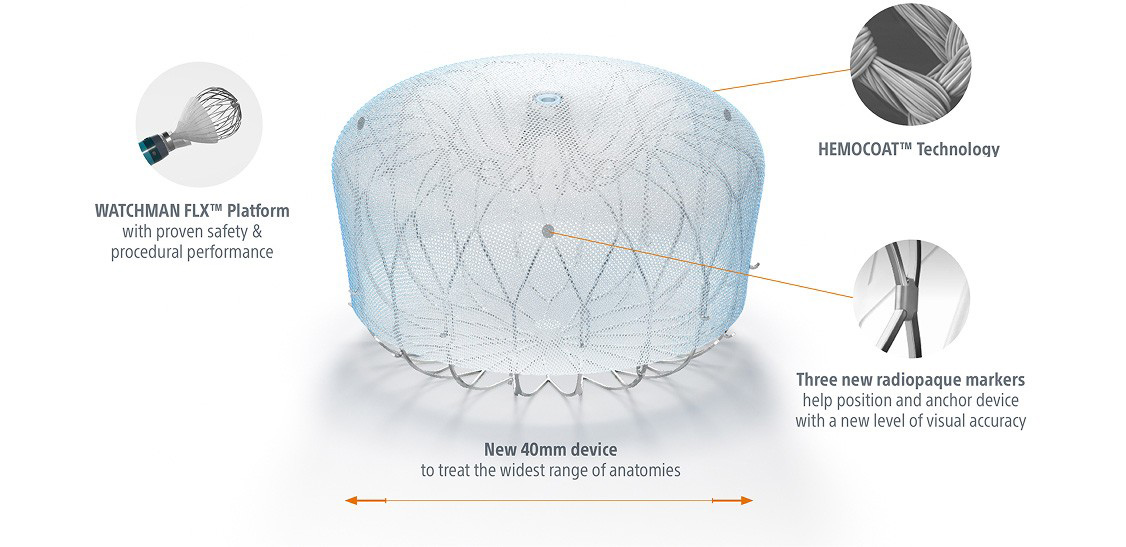
Shaping the future of LAAC therapy



Learn about the OPTION randomized clinical trial
References
1. Kar, S., et al, Circulation, 2021.
2. Wagner et al., Biomaterials Science: An Introduction to Materials in Medicine, 4th Edition, 2020.
3. Boston Scientific Data on file.
4. Bench study performed under CT by Boston Scientific. Data on file.
CAUTION: The law restricts these devices to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device. Products shown for INFORMATION purposes only and may not be approved or for sale in certain countries. This material not intended for use in France. 2025 Copyright © Boston Scientific Corporation or its affiliates. All rights reserved.